Levocetirizine hydrochloride injection and preparation method thereof
A technology of levocetirizine hydrochloride and injection, which is applied in the direction of pharmaceutical formulations, medical preparations containing active ingredients, medical preparations without active ingredients, etc., and can solve problems such as poor stability, high levels of related substances, and easy generation of foreign matter, etc. problems, to achieve the effects of stability improvement, anti-allergic reaction, and inhibition of impact
Pending Publication Date: 2022-04-12
JIANGSU ALICORN PHARMATECH CO LTD
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
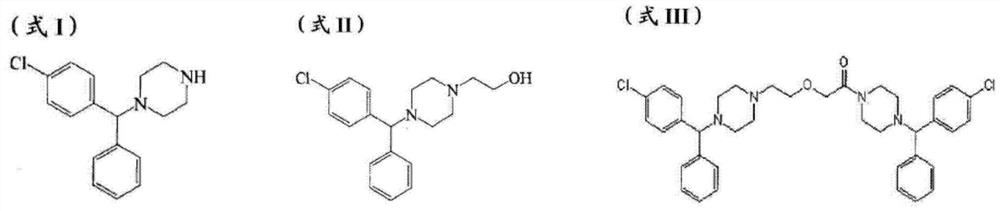
[0009] Levocetirizine injection containing sodium chloride was first disclosed in document WO2011003074, pH is 3-9; CN107961215A reported levocetirizine injection containing sodium chloride, pH is 4.9-5.2, after The inventor found
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
 Login to View More
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention relates to a levocetirizine injection and a preparation method thereof. The injection comprises an active component levocetirizine hydrochloride, cyclodextrin or a cyclodextrin derivative, an osmotic pressure regulator, a pH regulator and sterile water for injection. According to the levocetirizine hydrochloride injection prepared by the preparation method disclosed by the invention, related substances in stability lofting are less increased, the stability is remarkably improved, and the levocetirizine hydrochloride injection is widely applicable to patients with special constitution such as heart failure patients, pulmonary edema patients, encephaledema patients, intracranial pressure increase patients and gestational hypertension syndrome patients, can effectively resist anaphylaxis, and has good clinical application prospects. Therefore, clinical popularization is facilitated.
Description
technical field [0001] The invention belongs to the field of pharmaceutical preparations, in particular to a levocetirizine hydrochloride injection and a preparation method thereof. Background technique [0002] Allergic diseases, including allergic rhinitis, bronchial asthma, atopic dermatitis, and urticaria, are becoming serious public health problems. The frequency of allergic reactions is increasing due to changes in the climate environment and the increase in the types of food and drugs, so it is very important to develop drugs for the treatment of allergic reactions. [0003] Levocetirizine is the active optical isomer of cetirizine, which has a small dosage and less toxic and side effects than cetirizine. It has a long metabolic time and is a long-acting and highly selective peripheral H1 receptor antagonist. It can inhibit the release of various inflammatory mediators related to allergies and play a wide range of anti-inflammatory effects. It has no cardiotoxicity a...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): A61K9/08A61K31/495A61K47/69A61P37/08
Inventor 陆平波渠文静董海红赵青
Owner JIANGSU ALICORN PHARMATECH CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com