Gastric cancer prognosis biomarker and application thereof
A biomarker and prognostic technology, applied in biological testing, biomaterial analysis, instruments, etc., can solve the problems of single-cell phenotype monitoring that cannot CTC, cannot achieve accurate monitoring, etc., and achieve a minimally invasive, safe and The effect of improving the specimen acquisition process and predicting overall survival
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1 Separation and identification method of patient sample
[0025] A total of 92 patients with advanced gastric cancer who were treated in the Department of Gastroenterology and Oncology of Peking University from November 2017 to May 2021 were prospectively enrolled. Among them, 52 were male patients and 40 were female patients. 69 patients were ≤60 years old and 23 patients were >60 years old. All patients were diagnosed with advanced gastric or gastroesophageal junction malignant tumors by pathological diagnosis. The diagnosis of distant metastases was advanced gastric cancer by MRI and CT. Gastric cancer patients pathologically diagnosed as HER2-positive received chemotherapy combined with trastuzumab-based standard therapy; patients pathologically diagnosed as HER2-negative received taxane plus platinum-based chemotherapy. All enrolled patients have signed the informed consent.
[0026] 1. Take 6 mL of venous blood from patients with gastric cancer or co...
Embodiment 2
[0033] Example 2 CLDN18.2 + Determination of CTC
[0034] 1. CLDN18.2 + CTC identification criteria:
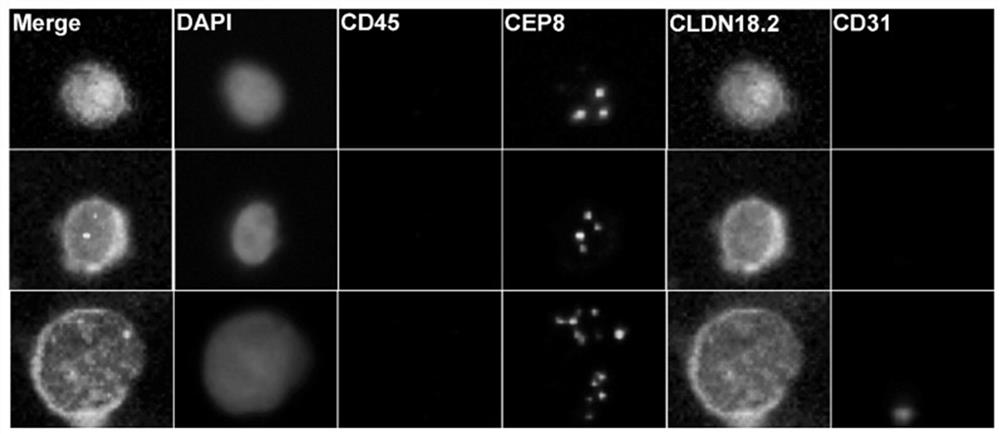
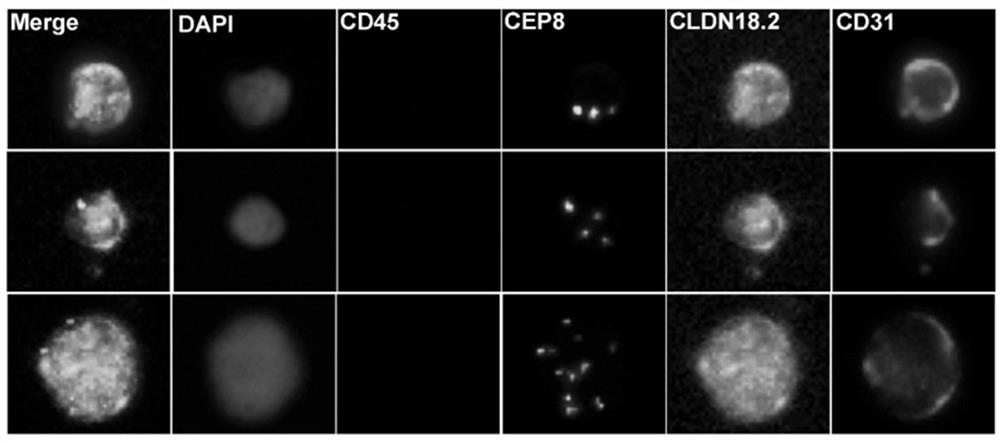
[0035] After completing the smart scan, the CLDN18.2 + CTCs were identified, and the chromosome 8 aneuploid amplified cells with negative expression of CD45, negative expression of CD31 and positive expression of CLDN18.2 were defined as CLDN18. + CTC); define CD45 negative, CD31 positive and CLDN18.2 positive chromosome 8 aneuploid amplified cells as CLDN18.2 + Circulating Endothelial Cells (CECs).
[0036] 2. CLDN 18.2 exists + Identification of gastric cancer patients with CTC or CEC:
[0037] After completing a single CLDN18.2 + After the identification of CTC and CEC, the total CLDN18.2 in the peripheral blood of each patient with gastric cancer + Count the number of CTC or CEC. Presence of ≥1 CLDN18.2 in peripheral blood + Gastric cancer patients with CTCs or CECs were defined as CLDN18.2 CTC or CEC-positive gastric cancer patients, and vice versa as CL...
Embodiment 3
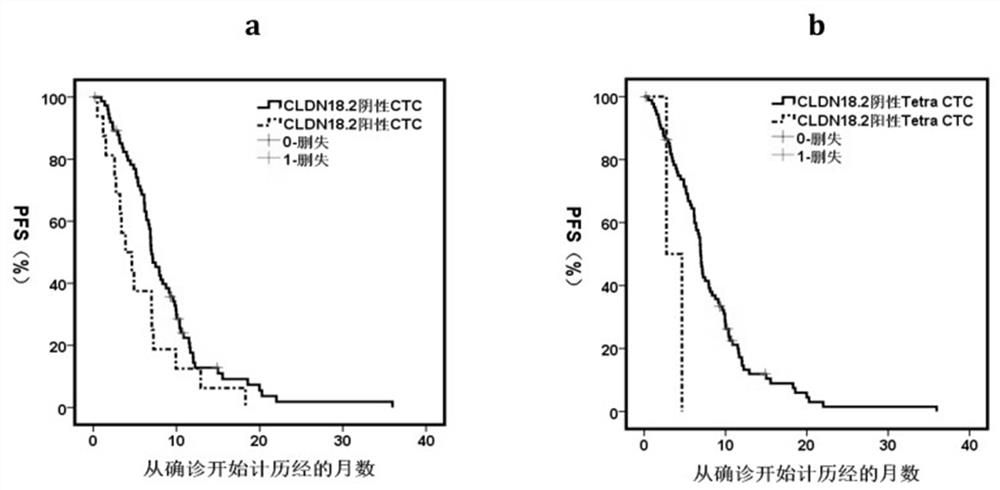
[0045] Example 3 CLDN18.2 in other samples of patients + The determination of CTC and its relationship with patient survival time (Overall Survival, OS)
[0046] In addition to detecting CLDN18.2-positive CTCs in peripheral blood, the detection method of the present invention can also detect CLDN18.2-positive tumor-related cells in ascites of patients with peritoneal metastatic gastric cancer and use the detected CLDN18.2 Positive for prognosis prediction in gastric cancer patients with peritoneal metastases.
[0047] In malignant tumors, overall survival time is a key indicator used to evaluate the prognosis of cancer patients. OS refers to the time from the start of treatment of malignant tumors to death from any cause in cancer patients. The shorter the OS time of the patients, the better the prognosis of tumor treatment, and vice versa, the worse the prognosis of tumor treatment. We prospectively enrolled 18 patients with intraperitoneal disseminated metastatic gastric ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com