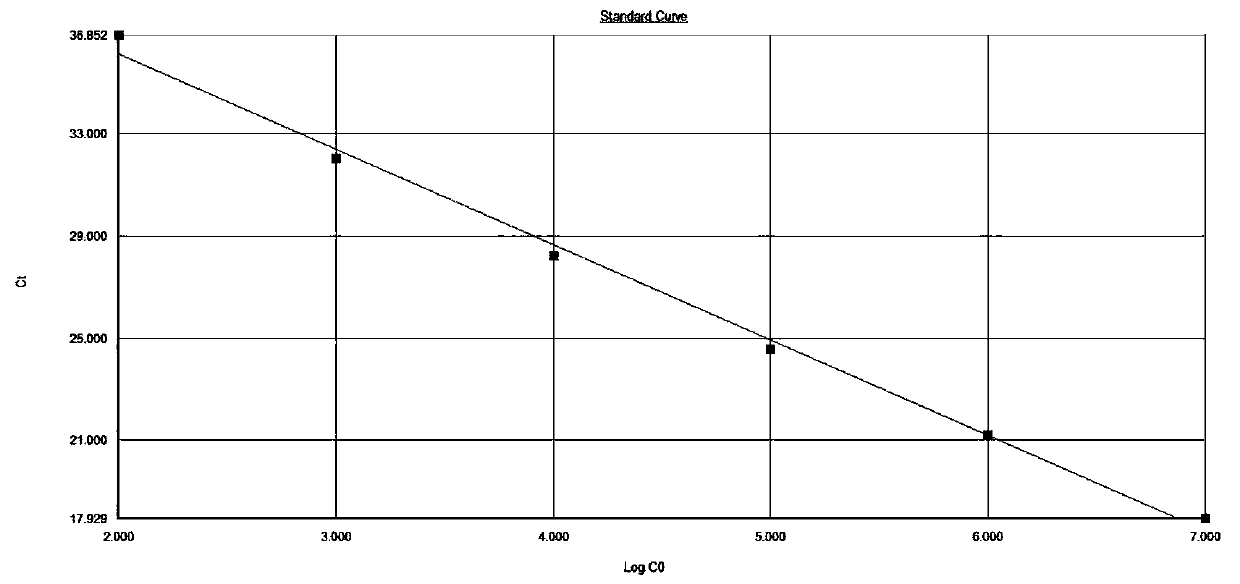
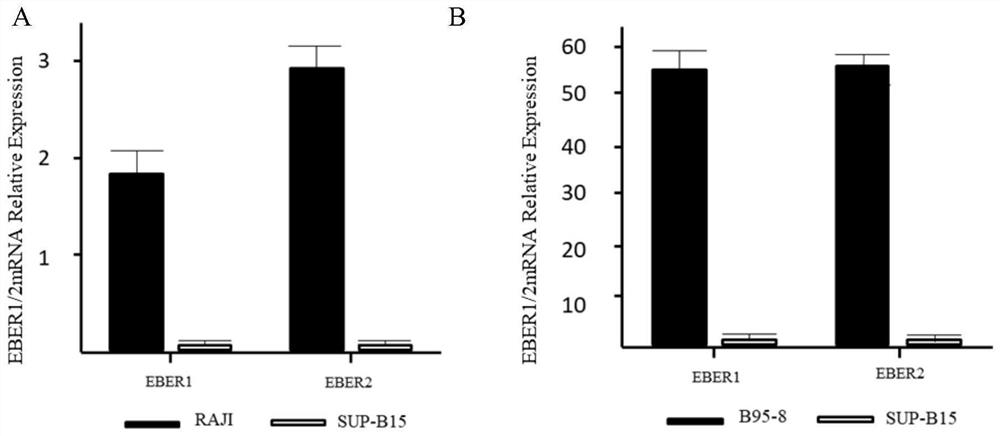
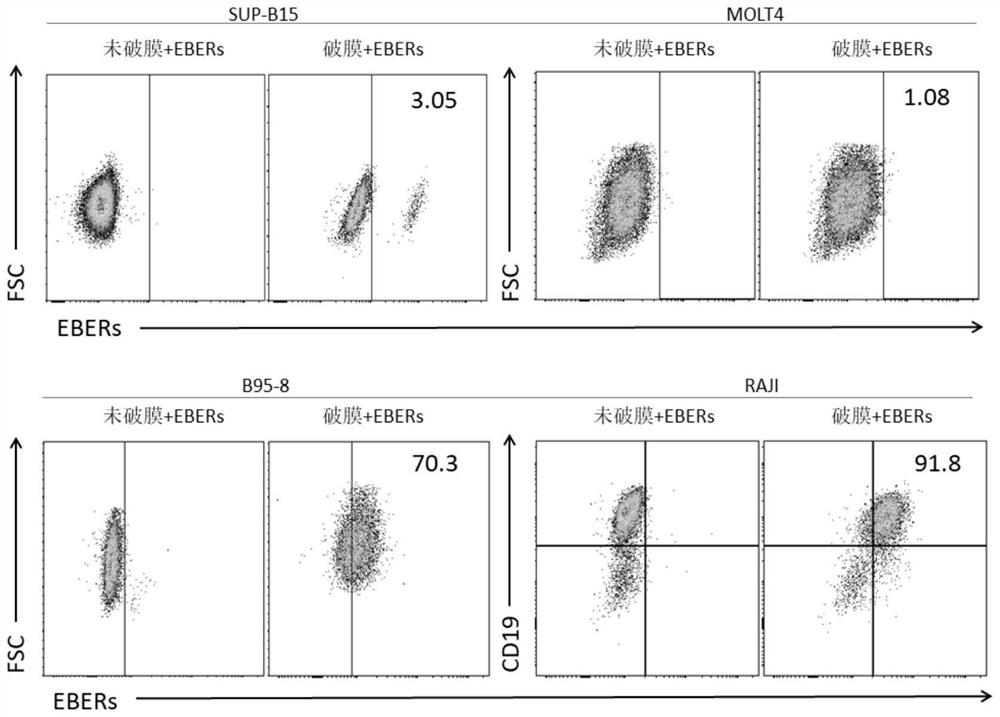
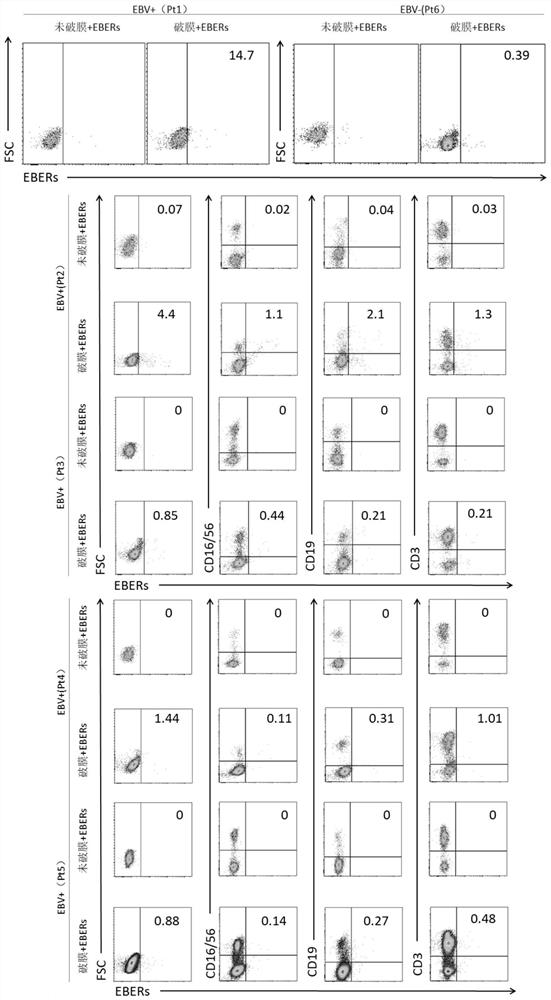
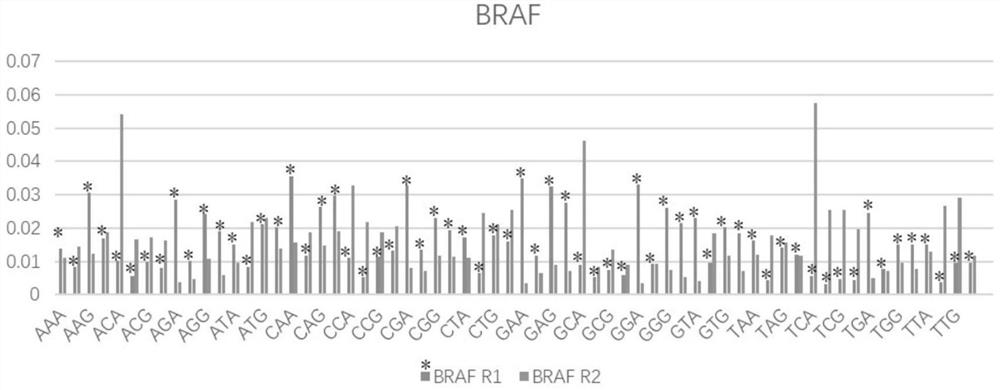
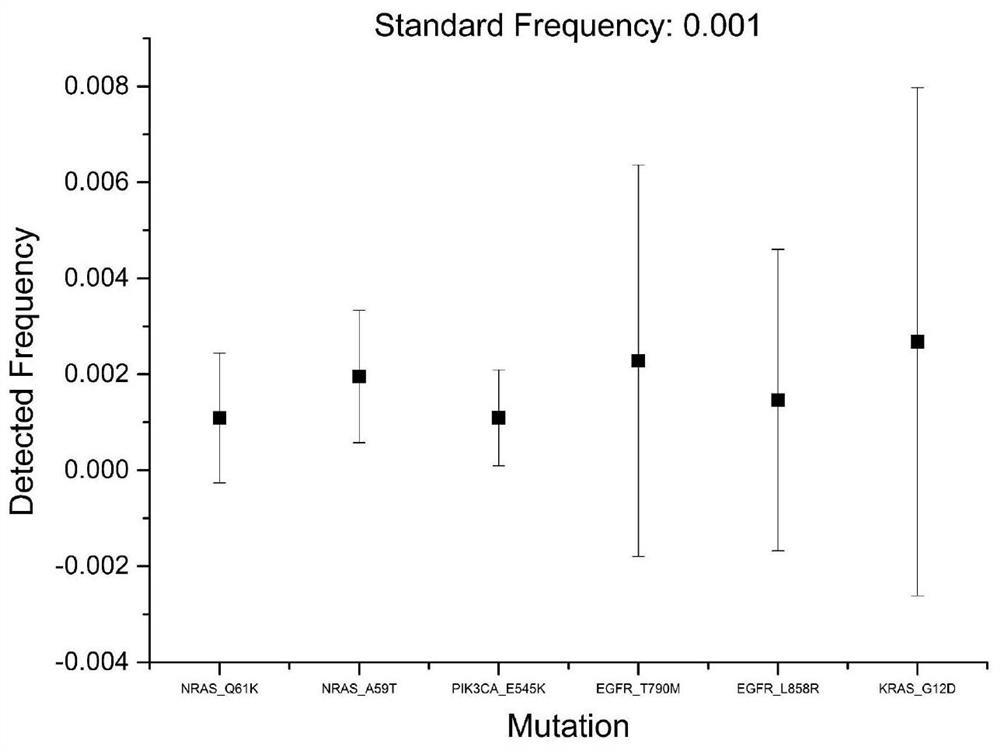
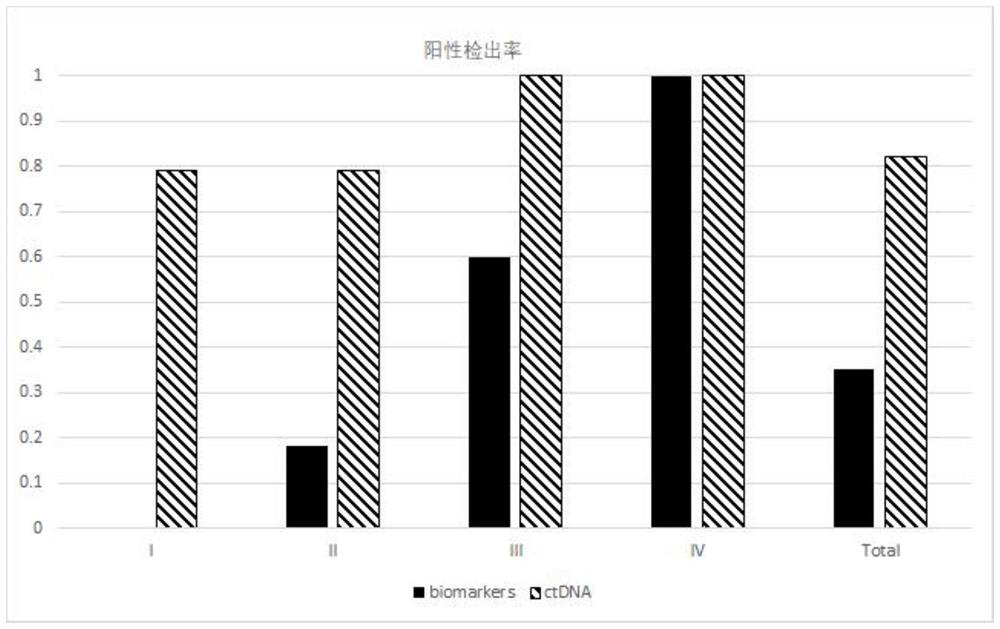
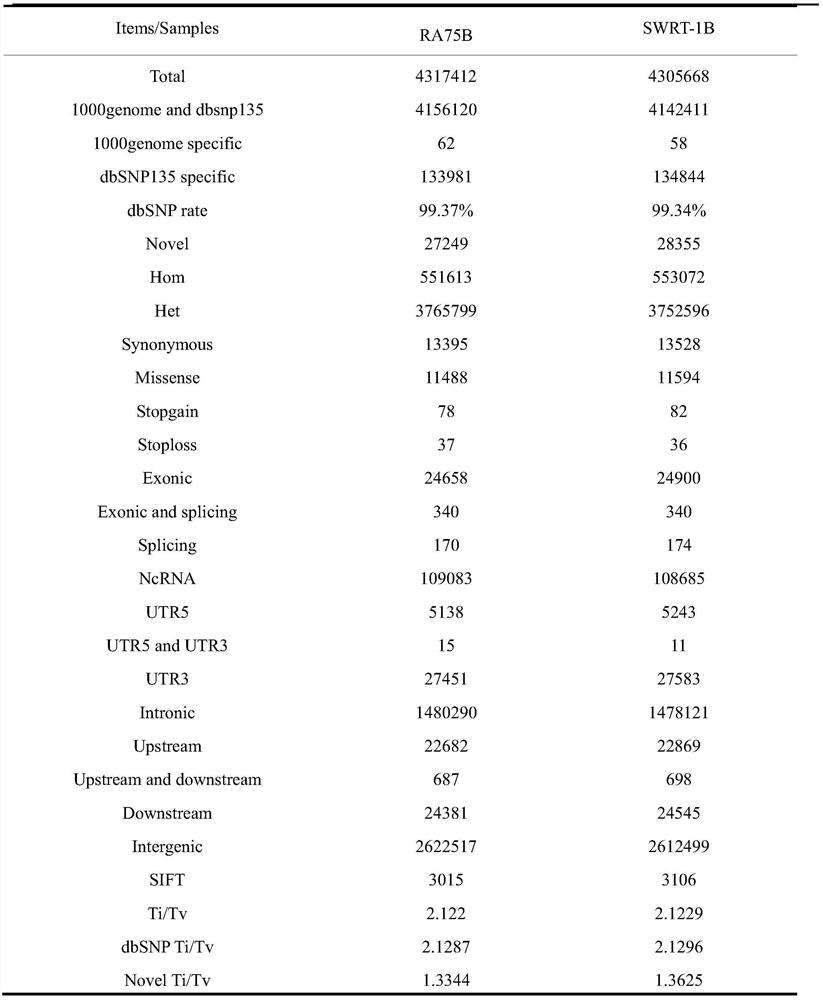
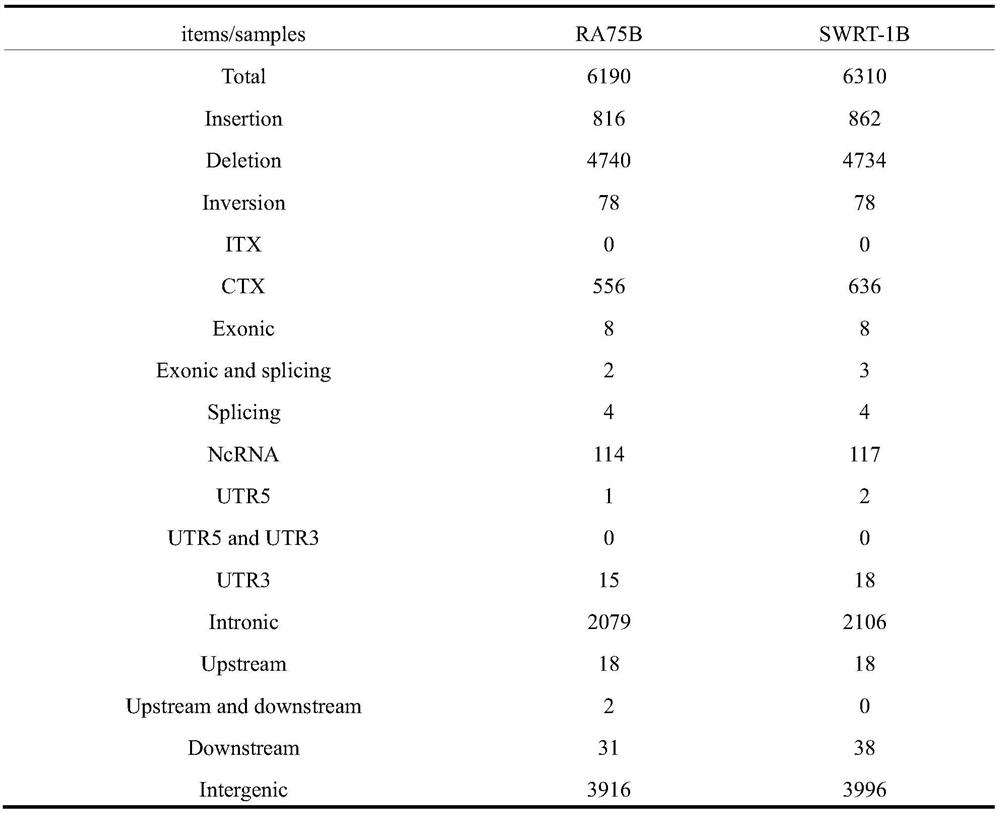
Patents
Literature
35 results about "Peripheral blood specimen" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
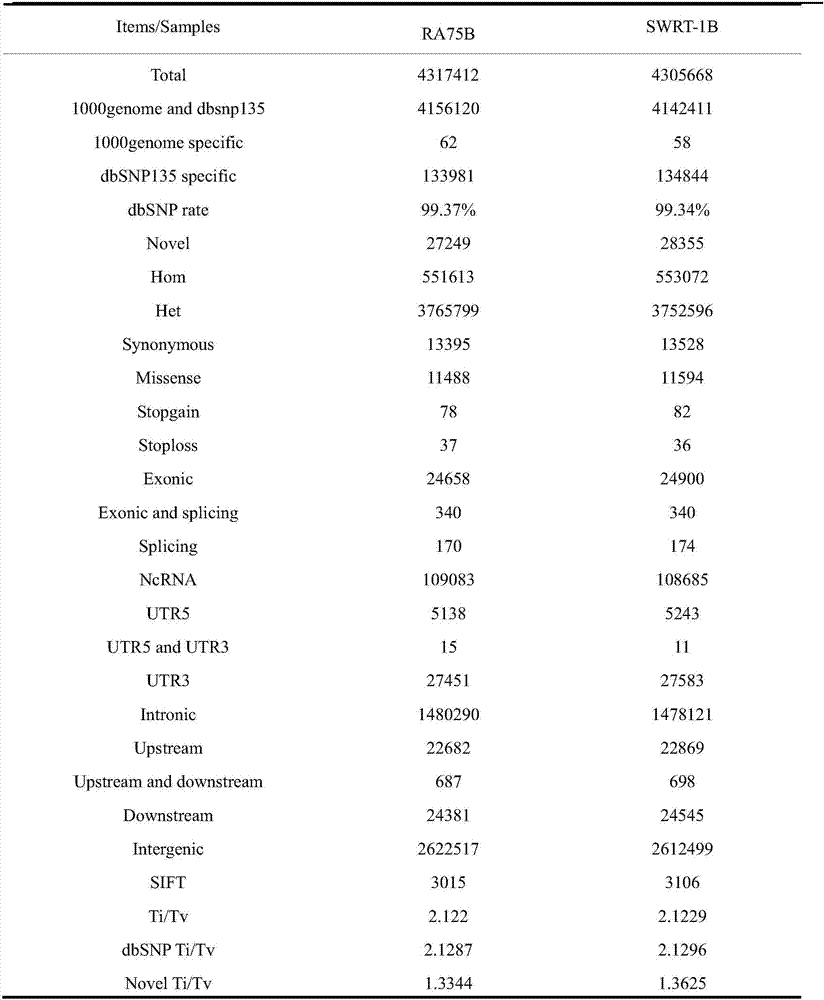
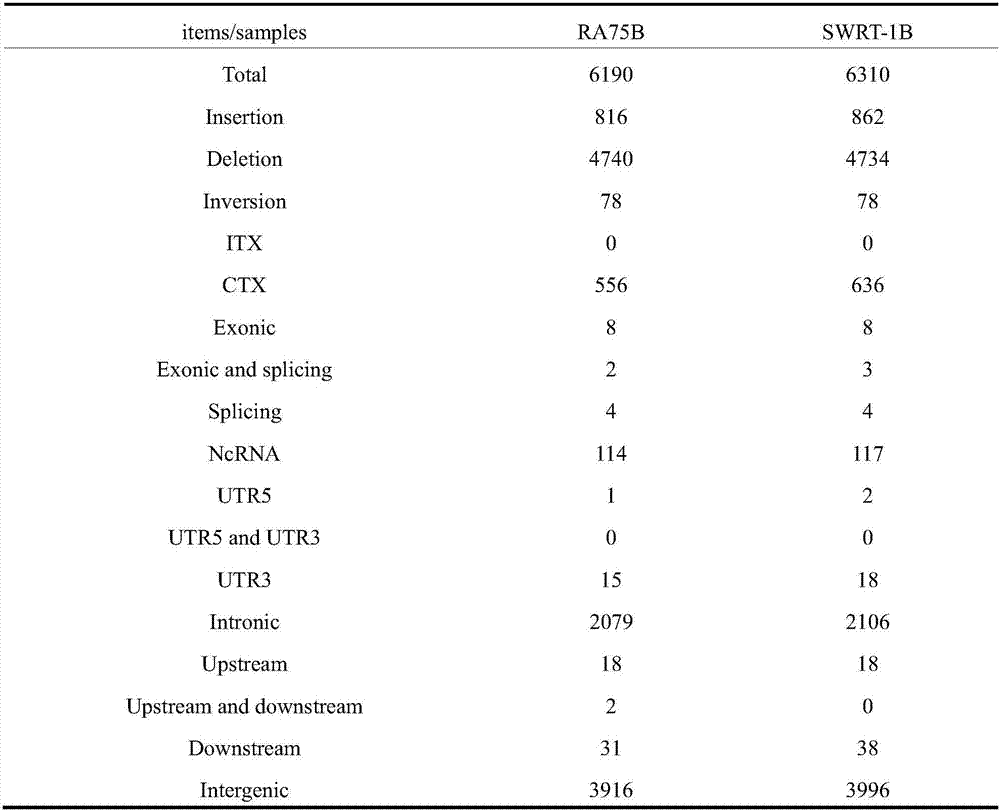
Detection method for non-small cell lung cancer peripheral blood circulating tumor cell PDL1 gene
ActiveCN106198984AGood clinical promotion valueReduce detectionMaterial analysisFiltrationPeripheral blood specimen
The invention provides a detection method for a non-small cell lung cancer peripheral blood circulating tumor cell PDL1 gene. The method comprises the steps that a, a peripheral blood sample from a non-small cell lung cancer is subjected to membrane filtration, and peripheral blood circulating tumor cells are obtained; b, a filter membrane obtained in the step a is fixed; c, the filter membrane obtained in the step b is detected in situ to determine the PDL1 expression condition; if PDL1 monochrome positive cells are detected in the step c, and HE dyeing is further used for identifying circulating tumor cells for the filter membrane.
Owner:上海立闻生物科技有限公司
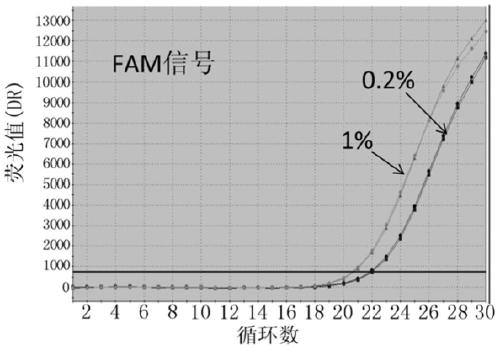
Probes, primers, detection system and kit for detecting mutations of EGFR gene
ActiveCN105349654AHigh sensitivityMeet the actual needs of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationEGFR Gene MutationBlood plasma
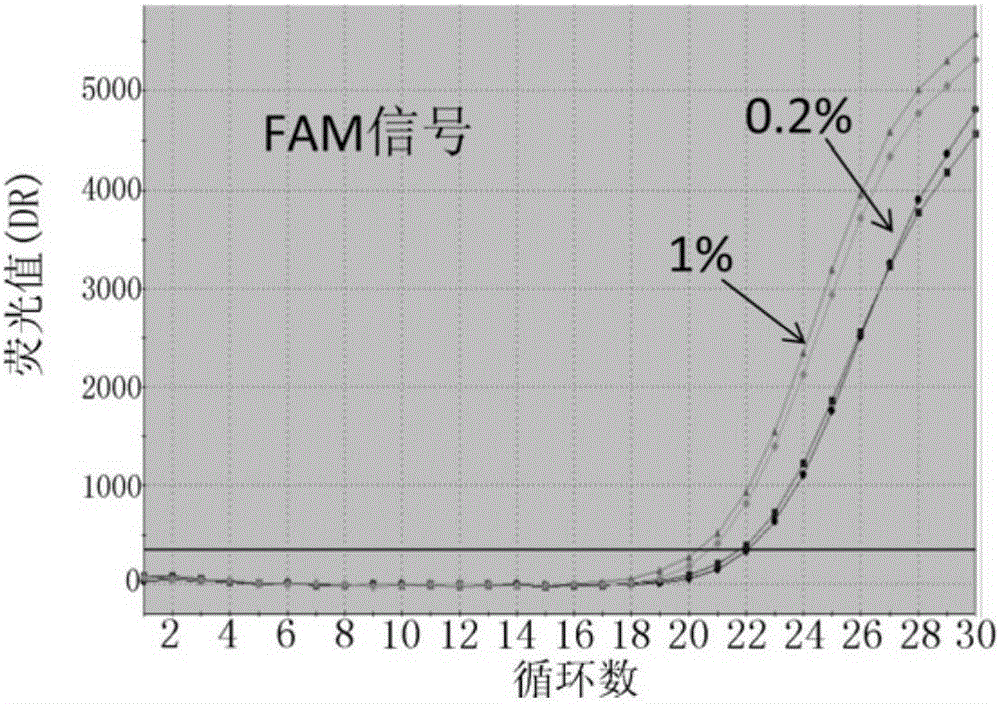
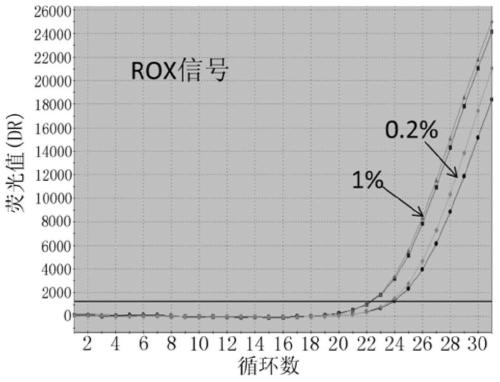
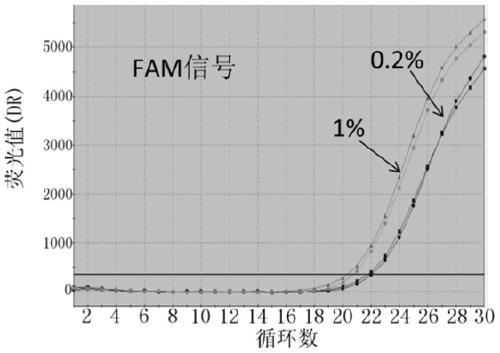
The invention discloses probes, primers, a detection system and a kit for detecting mutations of an EGFR gene, and the probes and the primers have the sequences of SEQ ID NO.1 to SEQ ID NO.24. The invention is characterized in that (1), the amplification efficiency is greatly improved to a largest extent; (2) the sensitivity is high, and the detection sensitivity can reach 0.2%; (3) compared with a digital PCR method, operations are simple, the cost is saved, and the clinical application scope is wide; (4) plasma samples with large reaction volume are detected, the DNA sampling quantity of the plasma samples becomes larger, the system is more stable, and the detection rate of the plasma samples is increased; (5) 25 mutations of the EGFR gene can be detected simultaneously in three reaction tubes, and results are intuitive and clear; (6) the detection speed is fast, and consumed time is only 1 / 2 that of the digital PCR; and (7) a detection method can detect peripheral blood samples, has convenient sampling, and can dynamically monitor curative effect of EGFR-TKI drugs on patients.
Owner:AMOY DIAGNOSTICS CO LTD
Diagnosis model and diagnosis kit for peripheral blood gene of liver cancer
The invention relates to a diagnosis model and a diagnosis kit for the peripheral blood gene of liver cancer. The diagnosis model comprises the expression level range indexes of 9 genes which are CXCR4, SPAG9, FOS, ANXA 1, CALR, IL8, PFN1, GPC3 and HGF; the kit comprises reagents which can detect the difference of expression levels of the 9 genes, namely the CXCR4, SPAG9, FOS, ANXA 1, CALR, IL8, PFN1, GPC3 and HGF from those of healthy people; and preferably, the reagents comprise an RNA extraction reagent and a multi-RT-PCR reagent, wherein the multi-RT-PCR reagent comprises SEQ ID No.1-18. The diagnosis accuracy of the diagnosis model established by the invention on five groups of peripheral blood samples is 76.0 percent.
Owner:GENERAL HOSPITAL OF PLA
Probes, primers and kit for detecting T790M mutation of EGFR gene
InactiveCN105112544AMeet the actual needs of rapid detectionHigh sensitivityMicrobiological testing/measurementDNA/RNA fragmentationPcr methodBlood plasma
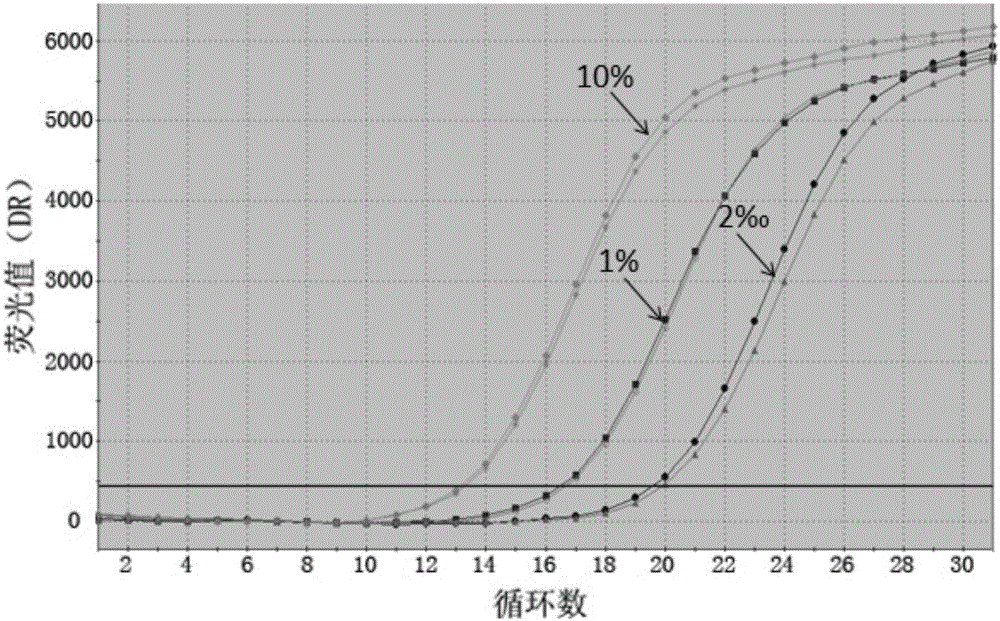
The invention discloses probes, primers and a kit for detecting a T790M mutation of an EGFR gene. The probes and the primers have the following sequence: SEQ ID NO: 01 to SEQ ID NO. 07. The probes, the primers and the kit have the following benefits: (1) SNP sites on the primers are designed as G / A merged basic groups, so that all efficiencies are compatible, and the amplification efficiency is improved; (2) the sensitivity is high, that is, the detection sensitivity can reach 2 permillage; (3) compared with those adopting a digital PCR method, the operation is simple, the cost is reduced, and the clinical application range is wide; (4) through blood plasma sample detection with a large reaction volume, the DNA loading quantity of blood plasma samples is increased, the detection system is more stable, and the detection rate of the blood plasma samples is improved; (5) the detection speed is high, that is, the detection process can be completed within only 120 minutes, and the time consumed in the detection process is only a half of that consumed according to the digital PCR method; (6) the probes, the primers and the kit, provided by the invention, can be utilized for detecting peripheral blood samples, so that convenient sampling and dynamic detection can be realized.
Owner:AMOY DIAGNOSTICS CO LTD
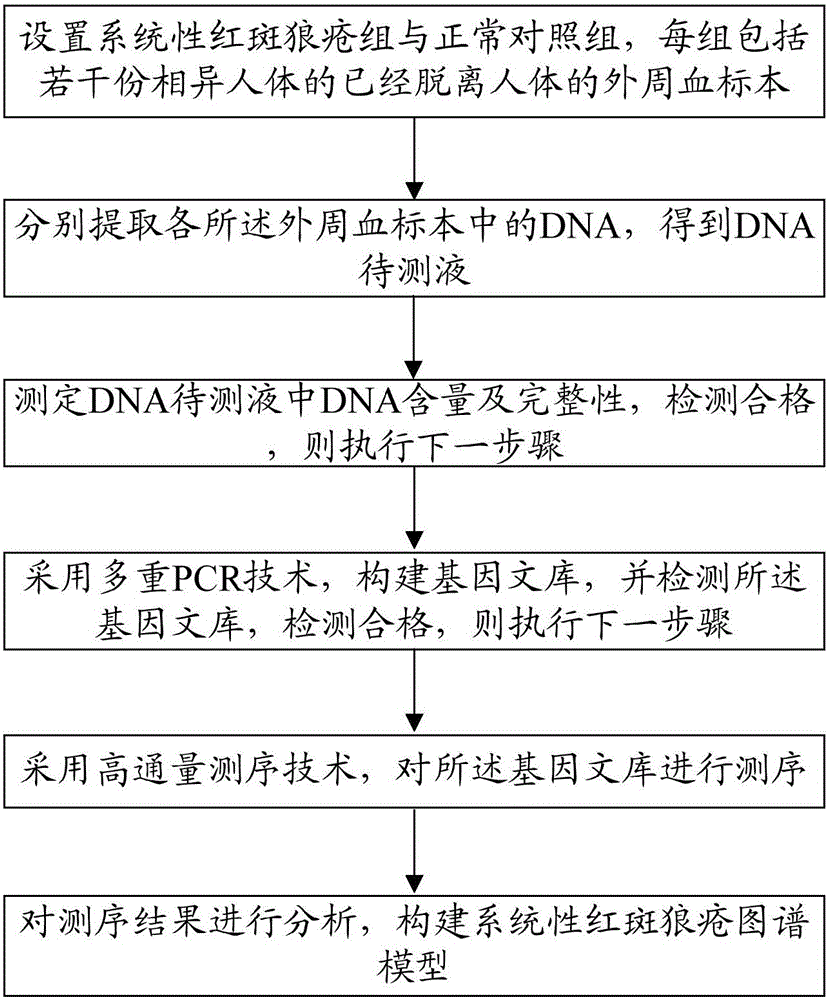
Construction method of systemic lupus erythematosus map model
ActiveCN105040111AReasonable designEfficient acquisitionMicrobiological testing/measurementLibrary creationHuman bodyPeripheral blood specimen
The invention relates to a construction method of a systemic lupus erythematosus map model. The construction method comprises: setting a systemic lupus erythematosus group and a control group, wherein each group comprises a plurality of peripheral blood samples isolated from human body and derived from different people; respectively extracting DNA from each peripheral blood sample to obtain DNA liquids to be detected; determining the DNA contents and the DNA integrity in the DNA liquids to be detected, detecting whether the sample is qualified, and performing the next step if the sample is qualified; constructing a gene library by using a multiplex PCR technology, detecting whether the gene library is qualified, and performing the next step if the gene library is qualified; carrying out sequencing on the gene library by using a high-throughput sequencing technology; and analyzing the sequencing results, and constructing the systemic lupus erythematosus map model. According to the construction method of the systemic lupus erythematosus map model of the present invention, the design is reasonable and feasible, and the SLE related information adopted as the middle results can be effectively obtained.
Owner:中国人民解放军联勤保障部队第九二四医院
A probe, primer, detection system and kit for detecting egfr gene mutation
ActiveCN105349654BHigh sensitivityMeet the actual needs of rapid detectionMicrobiological testing/measurementDNA/RNA fragmentationPeripheral blood specimenEGFR Gene Mutation
Owner:AMOY DIAGNOSTICS CO LTD
Biomarker of active pulmonary tuberculosis, and application of biomarker
ActiveCN111778341AEasy to collectEasy to handleMicrobiological testing/measurementMicroorganism based processesDiseaseNew medications
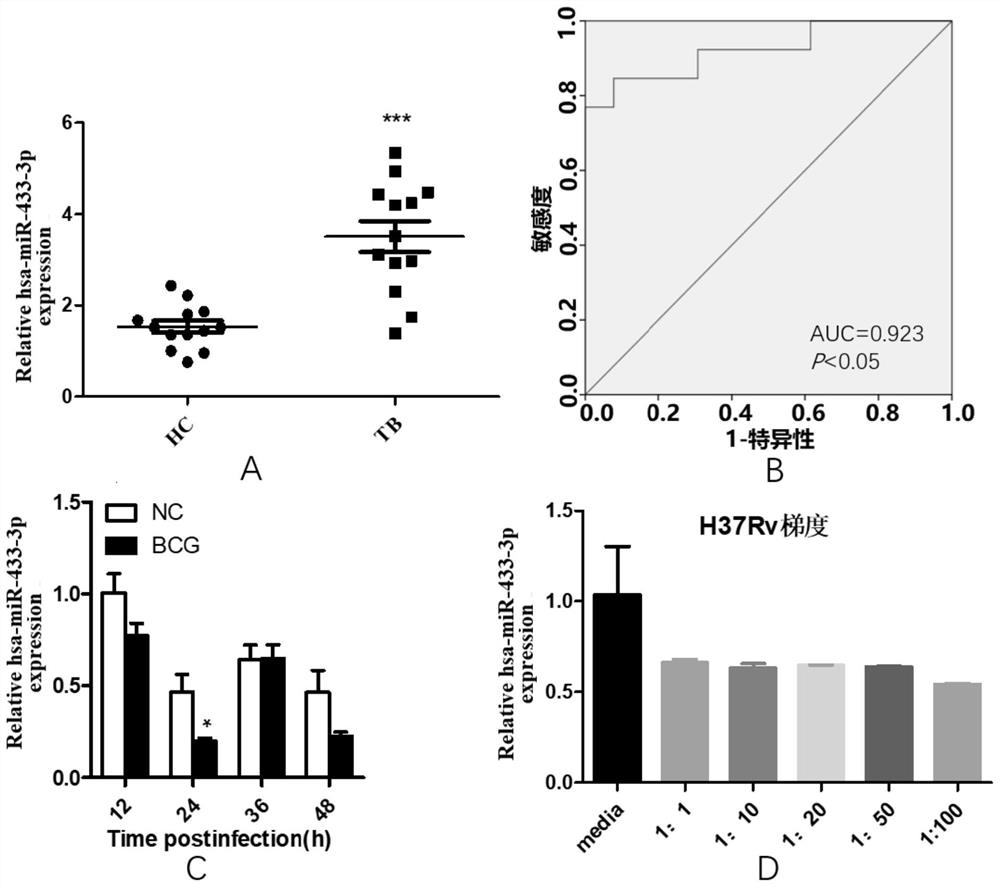
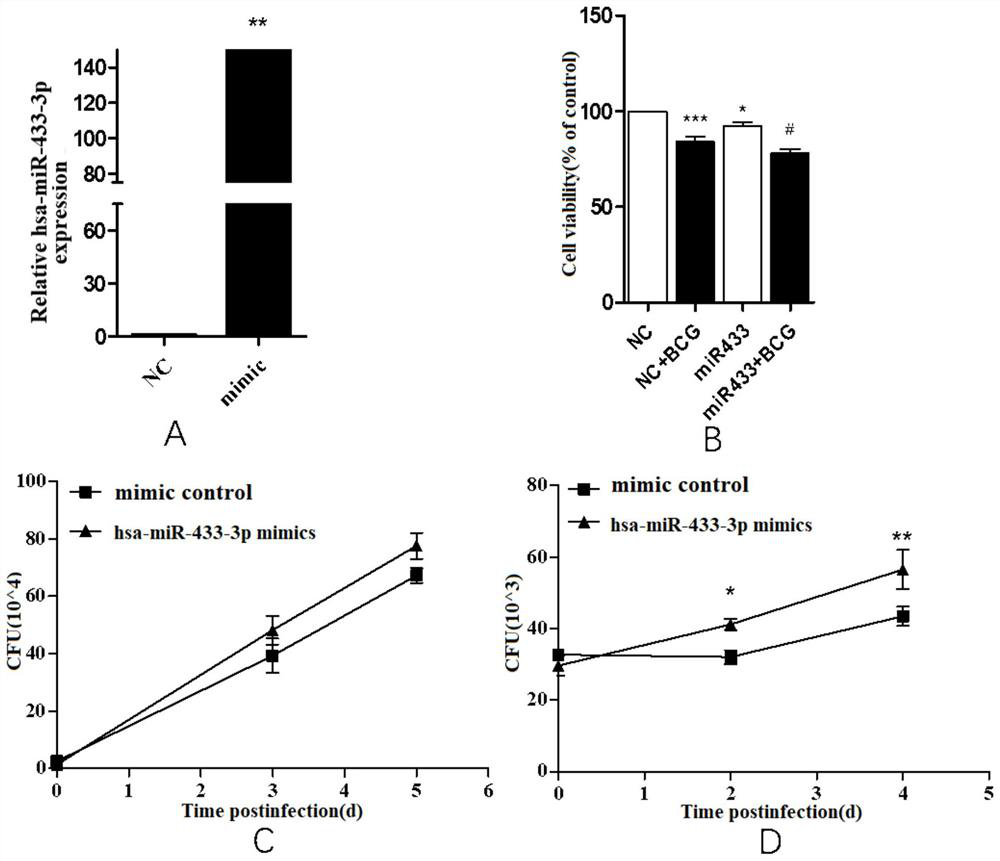
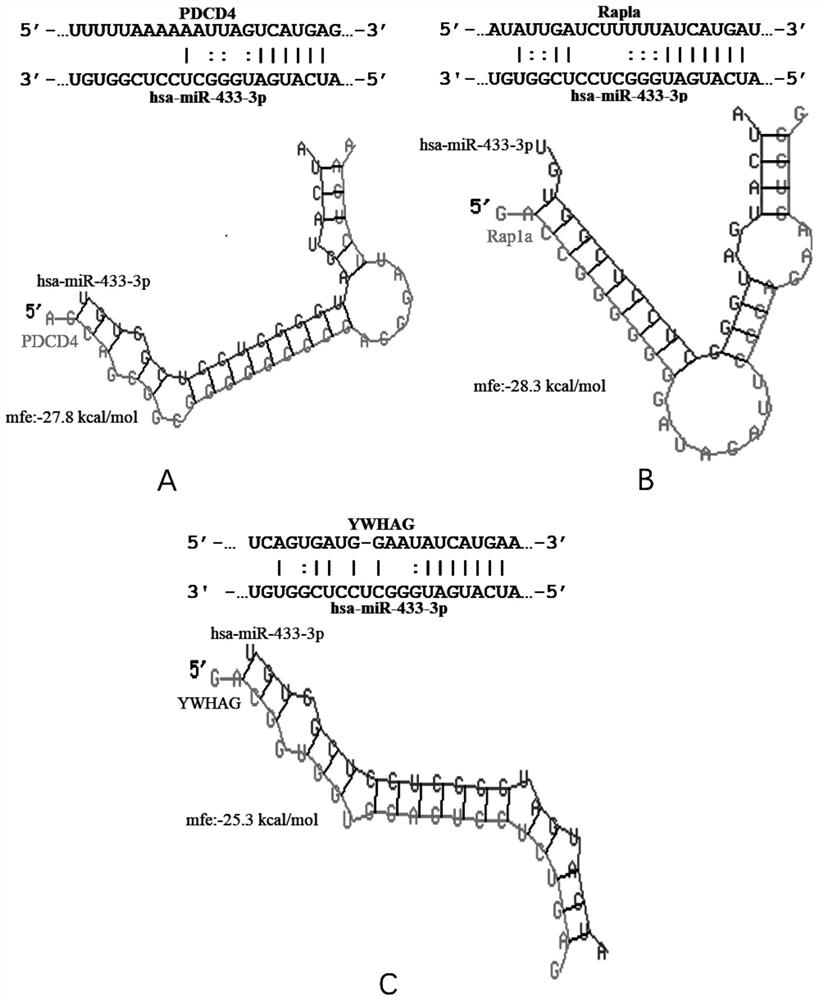
The invention provides a biomarker for active pulmonary tuberculosis, and relates to the technical field of biomarkers and therapeutic targets. The biomarker is hsa-miR-433-3p, and the hsa-miR-433-3pcan be used for the preparation of a diagnostic reagent for active pulmonary tuberculosis, and the active pulmonary tuberculosis diagnostic reagent prepared by using the hsa-miR-433-3p has the following advantages: the collection of a peripheral blood specimen is convenient, and the peripheral blood specimen is easy to treat; the detection consumed time is short, a result can be obtained quickly,and the rapid diagnosis of diseases is facilitated; and the detection cost is relatively low, and the economic burden of patients can be reduced. In addition, the hsa-miR-433-3p can also be used as atherapeutic target for active pulmonary tuberculosis, and the development of new drugs is facilitated.
Owner:GUANGDONG MEDICAL UNIV
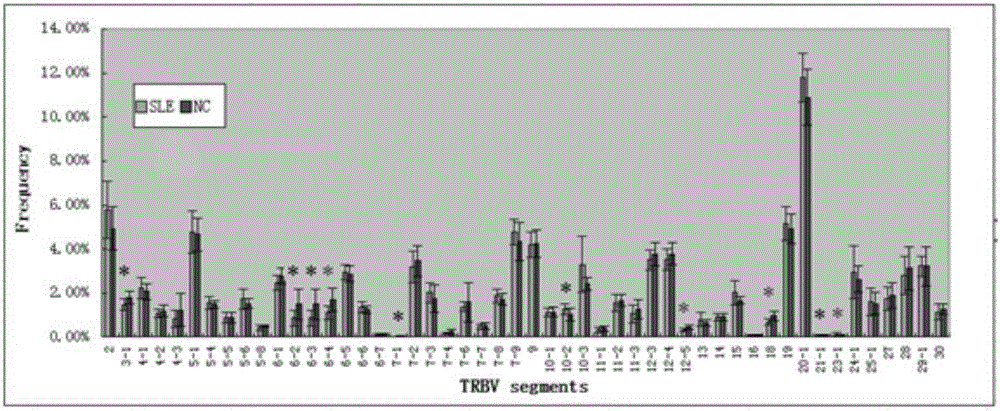
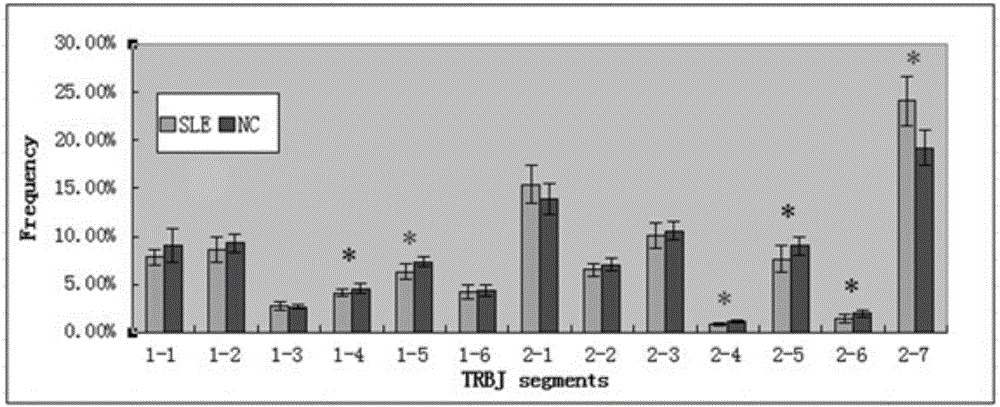
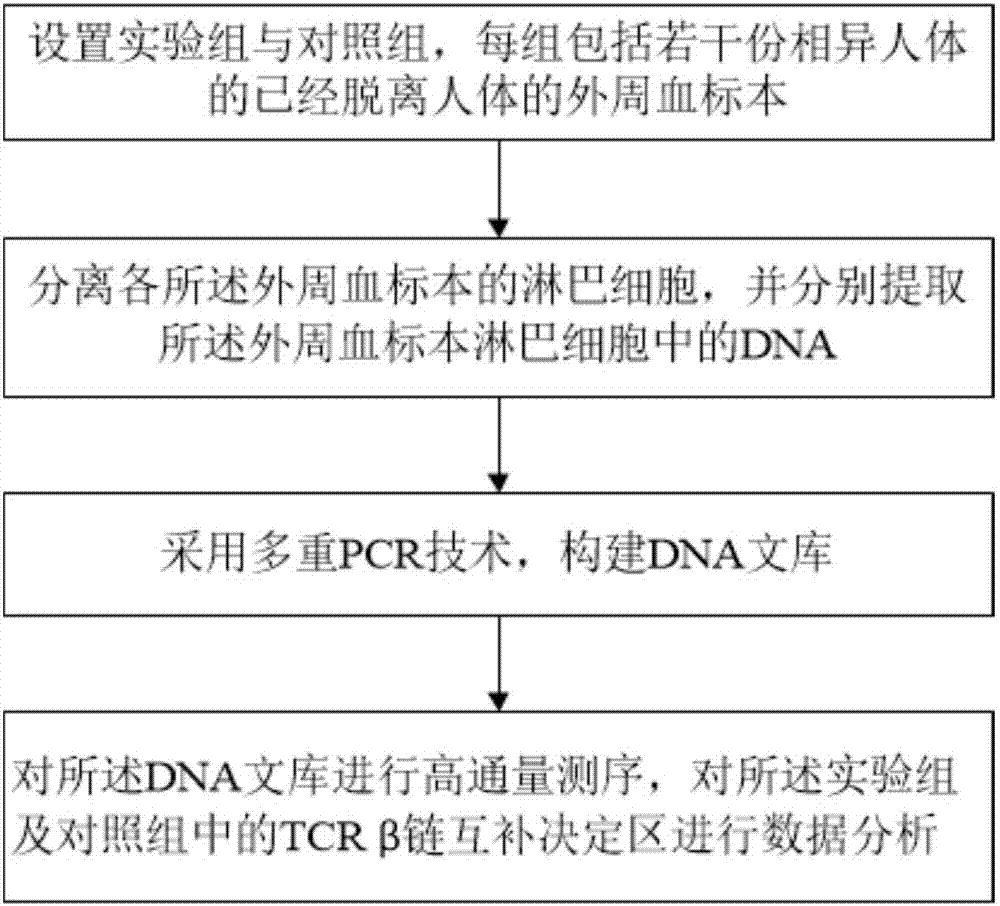
Method for treating complementary determining regions 3 (CDRs3) of T cell antigen receptor beta chains
InactiveCN107345240AImprove short termHigh activityMicrobiological testing/measurementImmunosuppressive drugComplementarity determining region
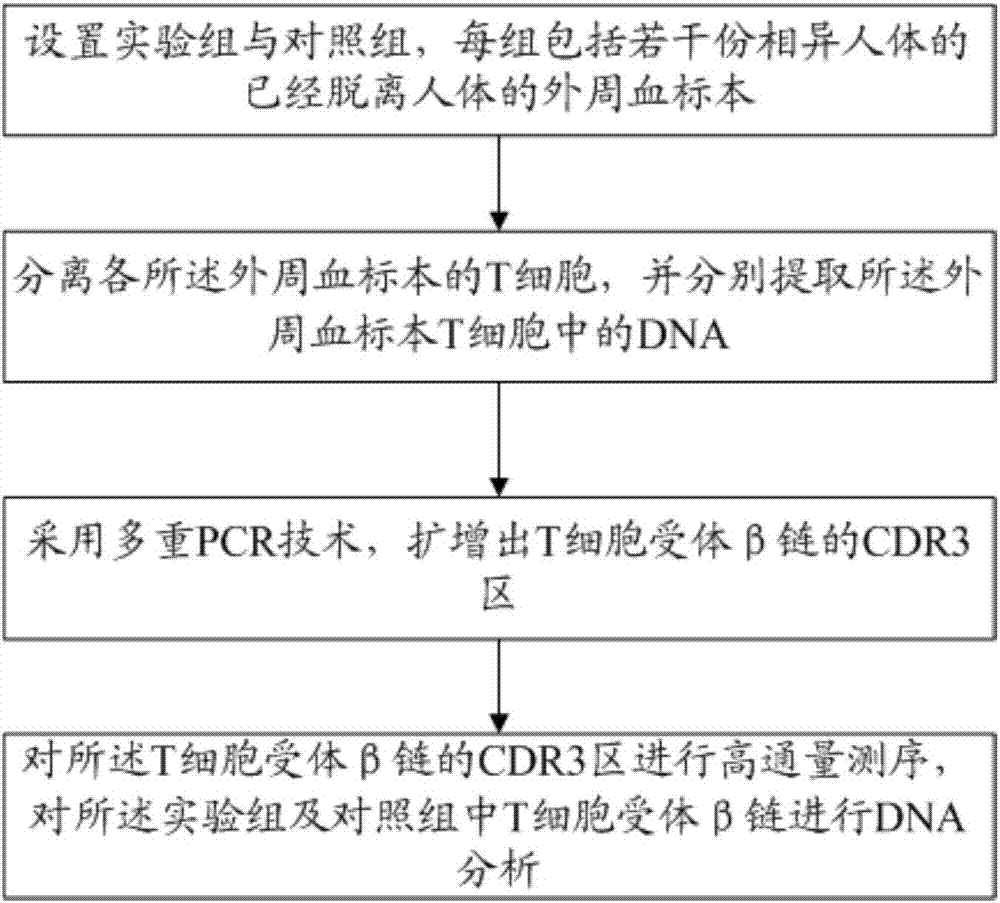
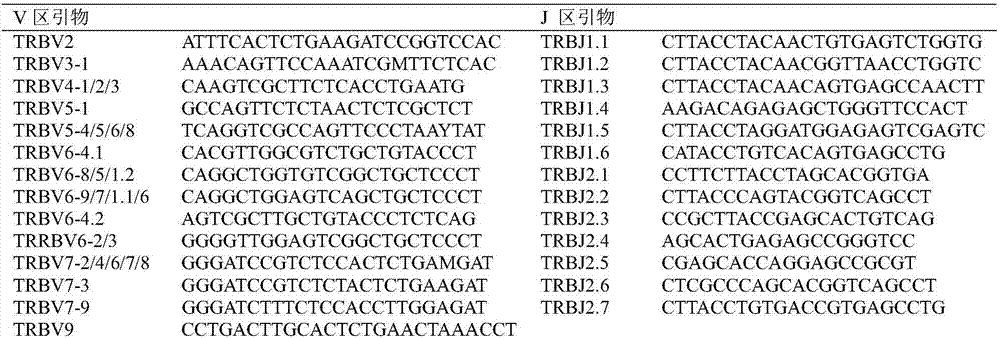
The invention relates to a method for treating complementary determining regions 3 (CDRs3) of T cell antigen receptor beta chains. The method comprises the following steps: setting an experimental group and a control group, wherein each group comprises a plurality of parts of peripheral blood samples which are dissimilar to a human body and get away from the human body; separating T cells of the peripheral blood samples, and respectively extracting deoxyribonucleic acid (DNA) in the T cells of the peripheral blood samples; adopting a multiplex polymerase chain reaction (PCR) technique to amplify the CDRs3 of the T cell antigen receptor beta chains; carrying out high-throughput sequencing on the CDRs3 of the T cell antigen receptor beta chains, and carrying out DNA analysis on the T cell antigen receptor beta chains in the experimental group and the control group. The method for treating the CDRs3 of the T cell antigen receptor beta chains is reasonable and feasible in design, and can be used for effectively acquiring information, taken as intermediate results, about renal transplant recipients at the perioperative period and further verifying, analyzing and researching the information so as to help to study the pathogenesis of a rejection reaction and guide the individual rational application of immunosuppressive drugs, thus being beneficial to increment of short-term and long-term survival rate of renal transplant recipients after transplantation.
Owner:眭维国 +2
Myasthenia gravis detection kit with non-coding RNA Tmevpg1 as detection or diagnosis screening marker and application of kit
ActiveCN104561257AProfound clinical significanceFar-reaching promotionMicrobiological testing/measurementMyasthenia gravisPeripheral blood specimen
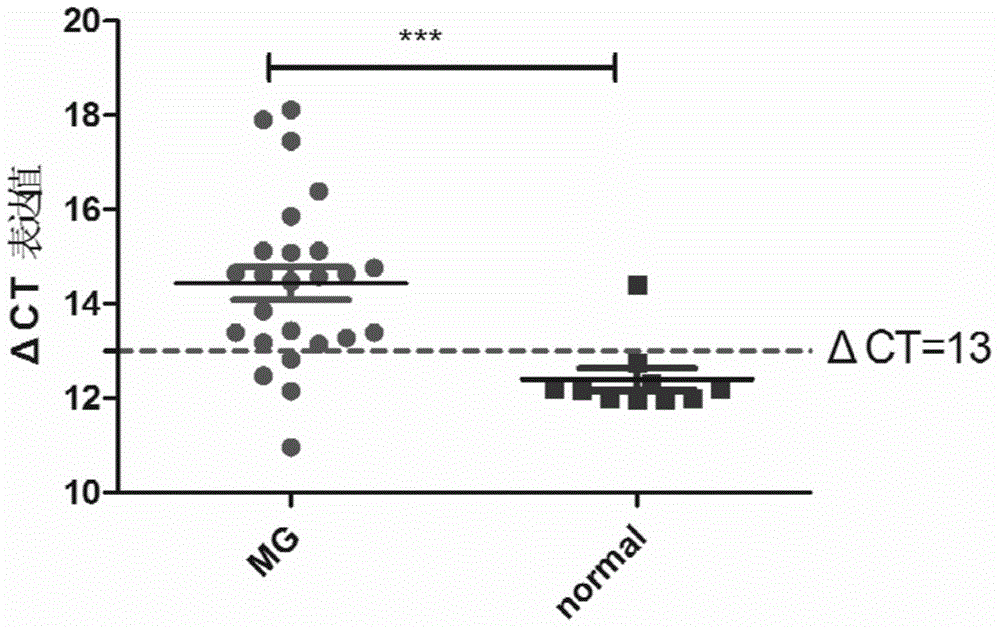
The invention discloses a myasthenia gravis detection kit with non-coding RNA Tmevpg1 as a detection or diagnosis screening marker and an application of the kit. The kit is applicable to myasthenia gravis screening and diagnosis by relatively quantitatively detecting the expression level of Tmevpg1 in the peripheral blood specimen of a myasthenia gravis patient.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
Breast cancer cell dyeing method, application thereof and dyeing kit
InactiveCN105223058AEasy to operateImprove accuracyPreparing sample for investigationKeratin AntibodyPeripheral blood specimen
The invention provides a breast cancer cell dyeing method. The method takes anti-pan cytokeratin antibody (AE1 / AE3) as the primary antibody, and adopts a horseradish peroxidase labeled rabbit antimouse antibody as the second antibody to label breast cancer cells, by means of diaminobenzidine (DAB) and hematoxylin dyeing, breast cancer cells can be dyed specifically. The invention also puts forward application of the dyeing method and a kit for carrying out the method. The method provided by the invention has the advantages of simple operation and good accuracy, can be used for detection of breast cancer micrometastatic cells in bone marrow blood and peripheral blood samples of breast cancer patients, and has important reference significance to prognosis of breast cancer.
Owner:JIANGSU PROVINCE HOSPITAL
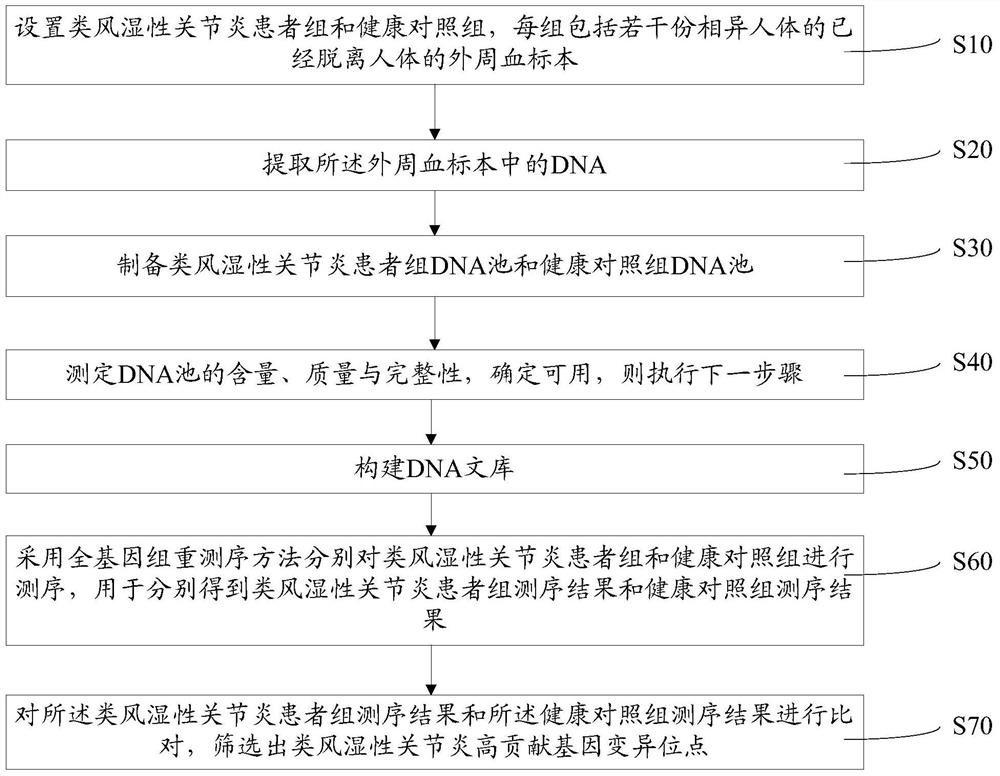
Method for screening high-contribution pathogenic gene of rheumatoid arthritis
ActiveCN107475351APlay a propelling roleMicrobiological testing/measurementHuman bodyScreening method
A method for screening a high-contribution pathogenic gene of rheumatoid arthritis comprises: setting a rheumatoid arthritis patient group and a healthy contrast group, each group comprising a plurality of peripheral-blood specimens, separated from the human body, of different human bodies; extracting DNA from the peripheral-blood specimens; preparing DNA pools of the rheumatoid arthritis patient group and the healthy contrast group; determining the content, quality and integrity of the DNA pools, and if the DNA pools are usable, carrying out the next step; building a DNA library; adopting a whole genome resequencing method to sequence the rheumatoid arthritis patient group and the healthy contrast group, and obtaining a rheumatoid arthritis patient group sequencing result and a healthy contrast group sequencing result; and comparing the rheumatoid arthritis patient group sequencing result with the healthy contrast group sequencing result, and screening high-contribution genovariation sites out. According to the method, high-contribution pathogenic genovariation sites are screened, and the method is beneficial for early stage treatment and prevention of rheumatoid arthritis.
Owner:戴勇 +1
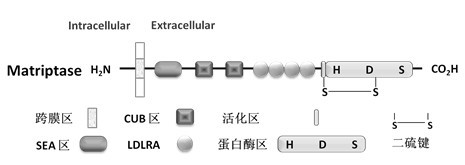
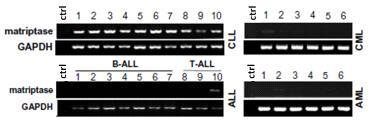
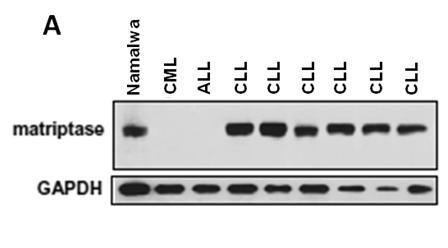
Application of Matriptase
The invention discloses an application of Matriptase. The Matriptase comprises a short intracellular segment, a transmembrane region, an SEA (sea urchin sperm protein, enteropeptidase, agrin domain) region, two CUB (Cls / Clr, urchin embryonic growth factor and bone morphogenetic protein-1) regions, four low density lipoprotein acceptor domains and a C-terminal trypsin-like protease region. The Matriptase is applied to diagnosis and treatment of chronic lymphocyte leukemia. By an experiment, bone marrows and peripheral blood specimens of normal people and leukemia sufferers are collected, and the detection result shows as follows: Matriptase mRNA and protein are highly expressed in the chronic lymphocyte leukemia sufferers, the expression level of Matriptase of the surface of lymphocyte can serve as a molecular marker for diagnosis of chronic lymphocyte leukemia, and the Matriptase can serve as a novel target for diagnosis and treatment of chronic lymphocyte leukemia.
Owner:SUZHOU UNIV
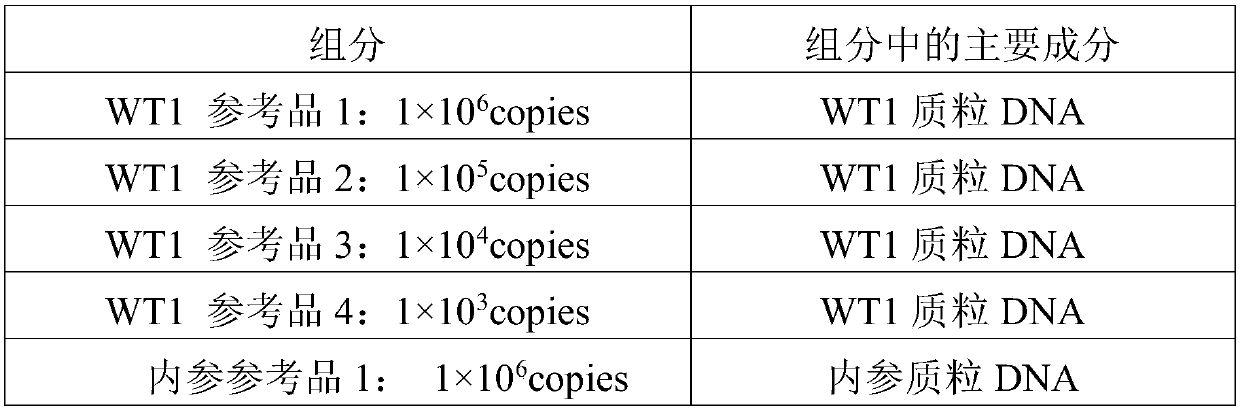
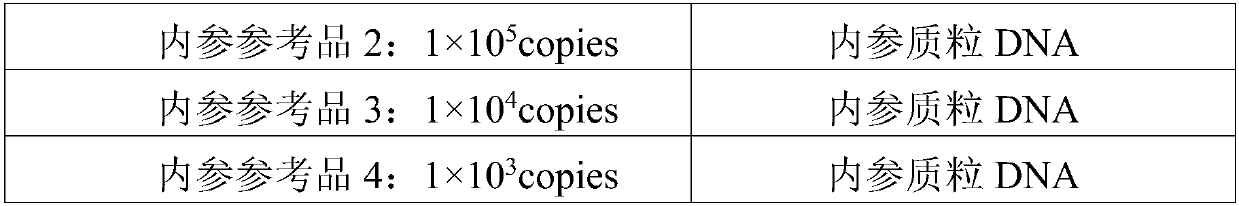
Reagent kit for detecting human WT1 fusion genes, and application method of reagent kit
PendingCN110551817AHigh minimum detectable amountWide linear rangeMicrobiological testing/measurementReverse transcriptasePeripheral blood specimen
The application of the invention relates to the field of detection of fusion genes, in particular to a reagent kit for detecting human WT1 fusion genes. According to the reagent kit disclosed by the application of the invention, reverse transcription is performed by a one-step method, namely that mRNA in a sample is subjected to reverse transcription to form cDNA through a specific primer and an enzyme, the cDNA obtained through reverse transcription directly enters a downstream fluorescent quantitation PCR, and RNA of a WT1 gene in a clinical peripheral blood specimen is subjected to quantitative detection, so that the reagent kit assists myoblastosis clinical diagnosis and guides relevant myoblastosis patients to choose clinical medicines, and has technical advantages of being high in specificity, high in accuracy and lowest in detection quantity.
Owner:苏州云泰生物医药科技有限公司
Method for treating complementary determining regions 3 (CDRs3) of B cell antigen receptor H chains
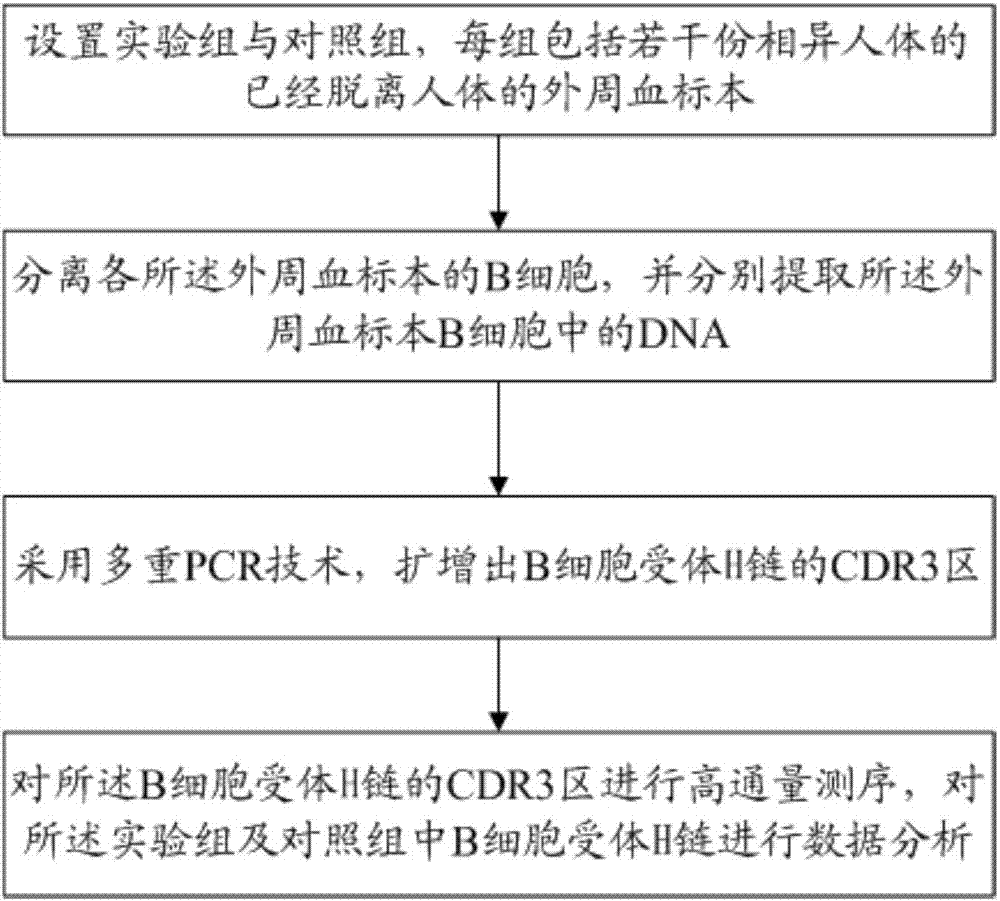
InactiveCN107345241ATo elucidate the pathogenesisDeepen understandingMicrobiological testing/measurementImmunosuppressive drugComplementarity determining region
The invention relates to a method for treating complementary determining regions 3 (CDRs3) of B cell antigen receptor H chains. The method comprises the following steps: setting an experimental group and a control group, wherein each group comprises a plurality of parts of peripheral blood samples which are dissimilar to a human body and get away from the human body; separating B cells of the peripheral blood samples, and respectively extracting deoxyribonucleic acid (DNA) in the B cells of the peripheral blood samples; adopting a multiplex polymerase chain reaction (PCR) technique to amplify the CDRs3 of the B cell antigen receptor H chains; carrying out high-throughput sequencing on the CDRs3 of the B cell antigen receptor H chains, and carrying out DNA analysis on the B cell antigen receptor H chains in the experimental group and the control group. The method for treating the CDRs3 of the B cell antigen receptor H chains is reasonable and feasible in design, and can be used for effectively acquiring information, taken as intermediate results, about renal transplant recipients at the perioperative period and further verifying, analyzing and researching the information so as to help to study the pathogenesis of a rejection reaction and guide the individual rational application of immunosuppressive drugs, thus being beneficial to improvement of the treatment of patients with end-stage renal disease.
Owner:眭维国 +3
PLC (Primary Liver Cancer) perioperative period liver transplantation patient circRNA differential expression profile map model as well as building method and building system thereof
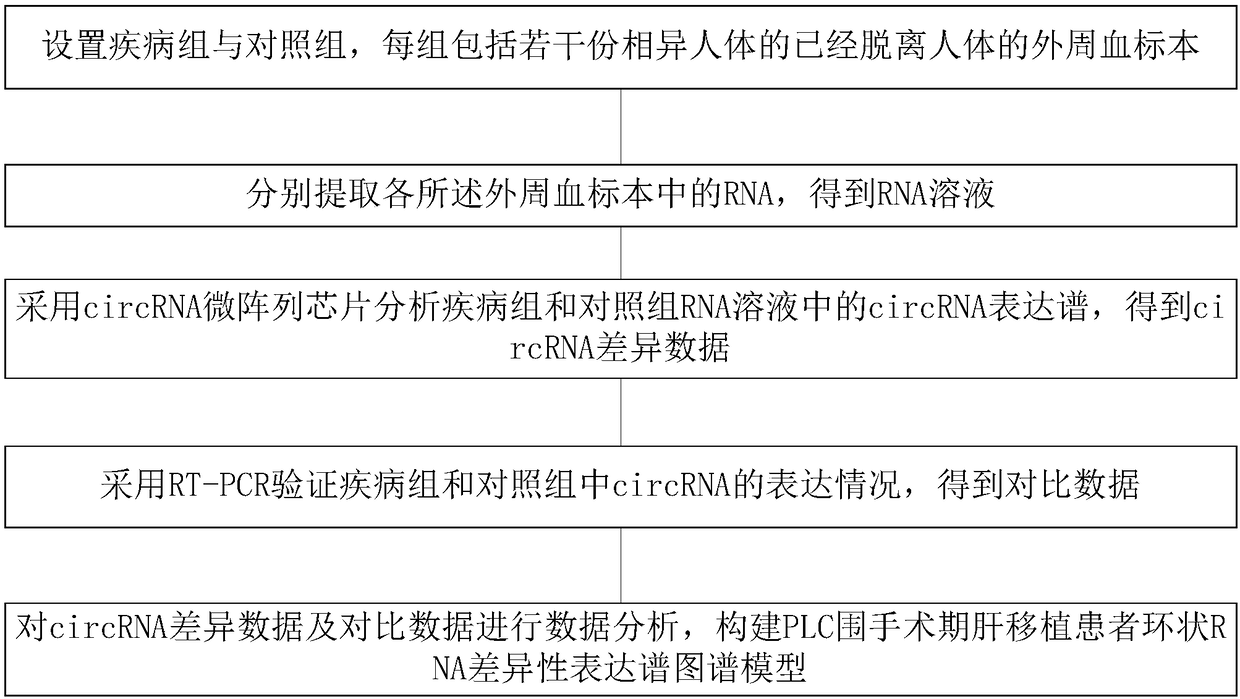
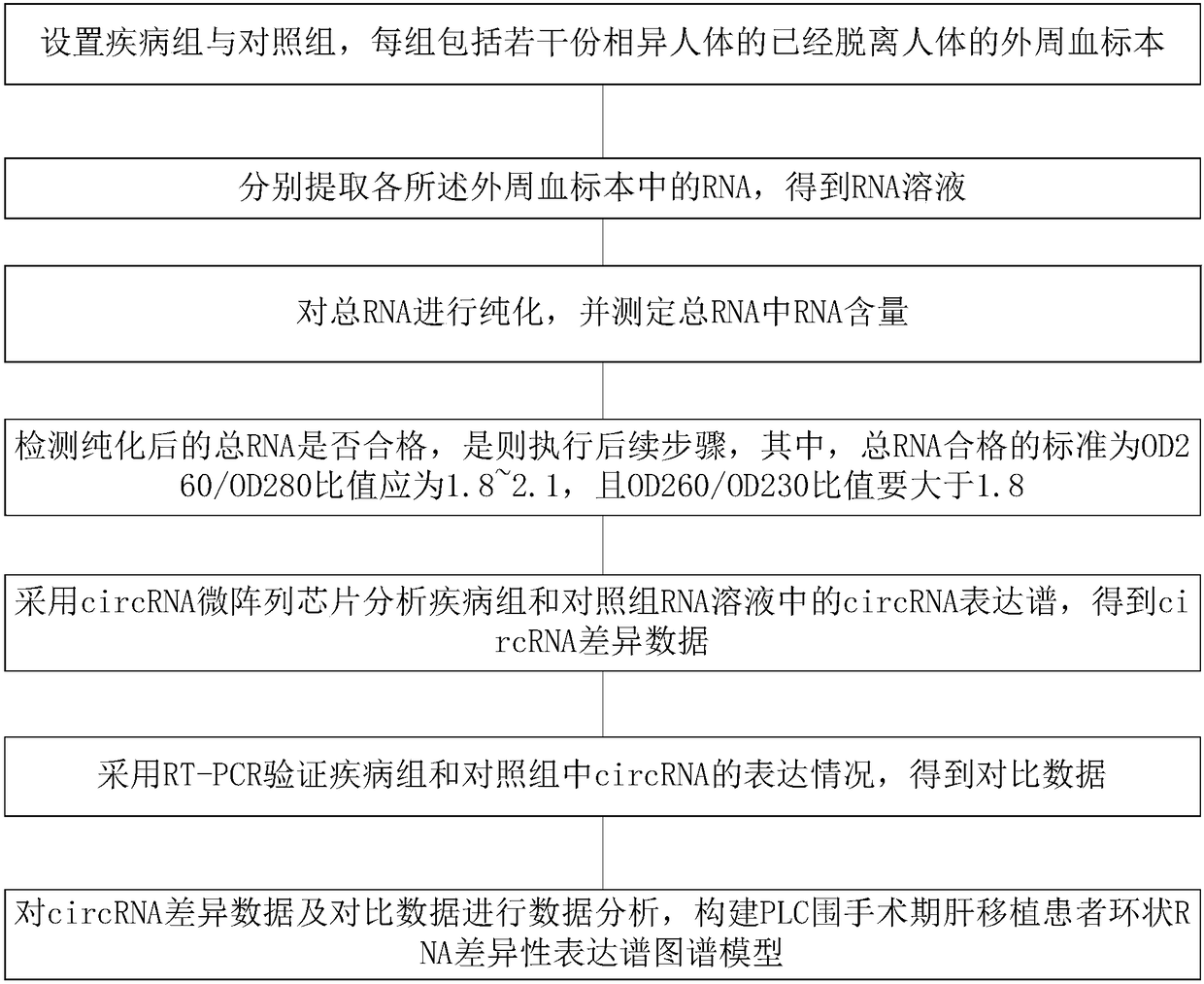
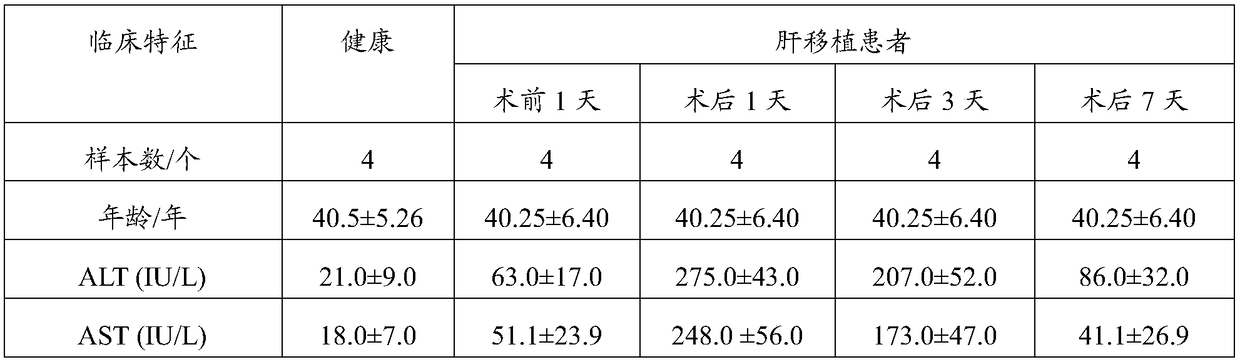
The invention discloses a PLC (Primary Liver Cancer) perioperative period liver transplantation patient circRNA differential expression profile map model as well as building method and building systemthereof. The building method comprises the following steps: setting a disease group and a control group, wherein each group includes a plurality of peripheral blood specimens of different human bodies, which are already separated from the human bodies; extracting RNAs respectively; analyzing circRNA expression profiles in RNA solutions of the disease group and the control group by adopting a circRNA microarray chip to obtain circRNA differential data; verifying the expression situations of circRNA in the disease group and the control group by adopting RT-PCR to obtain comparison data; performing data analysis on the circRNA differential data and the comparison data to build the PLC perioperative period liver transplantation patient circRNA differential expression profile map model. According to the building method, differentially-expressed circRNAs among a patient before liver transplantation, the patient subjected to liver transplantation and a healthy control are compared to screenPLC specifically-expressed circRNAs and circRNAs which are correlated with post-transplantation early liver function recovery, so that new information is provided for the early diagnosis, prognosis and treatment of PLC.
Owner:戴勇 +3
Detection method of pdl1 gene in peripheral blood circulating tumor cells of patients with non-small cell lung cancer
The invention provides a detection method for a non-small cell lung cancer peripheral blood circulating tumor cell PDL1 gene. The method comprises the steps that a, a peripheral blood sample from a non-small cell lung cancer is subjected to membrane filtration, and peripheral blood circulating tumor cells are obtained; b, a filter membrane obtained in the step a is fixed; c, the filter membrane obtained in the step b is detected in situ to determine the PDL1 expression condition; if PDL1 monochrome positive cells are detected in the step c, and HE dyeing is further used for identifying circulating tumor cells for the filter membrane.
Owner:上海立闻生物科技有限公司
Mixed gene for detecting fusion gene, standard plasmid, kit and preparation method thereof
ActiveCN108676848BImprove detection efficiencyImprove detection accuracyMicrobiological testing/measurementDNA/RNA fragmentationRUNX1RUNX1T1
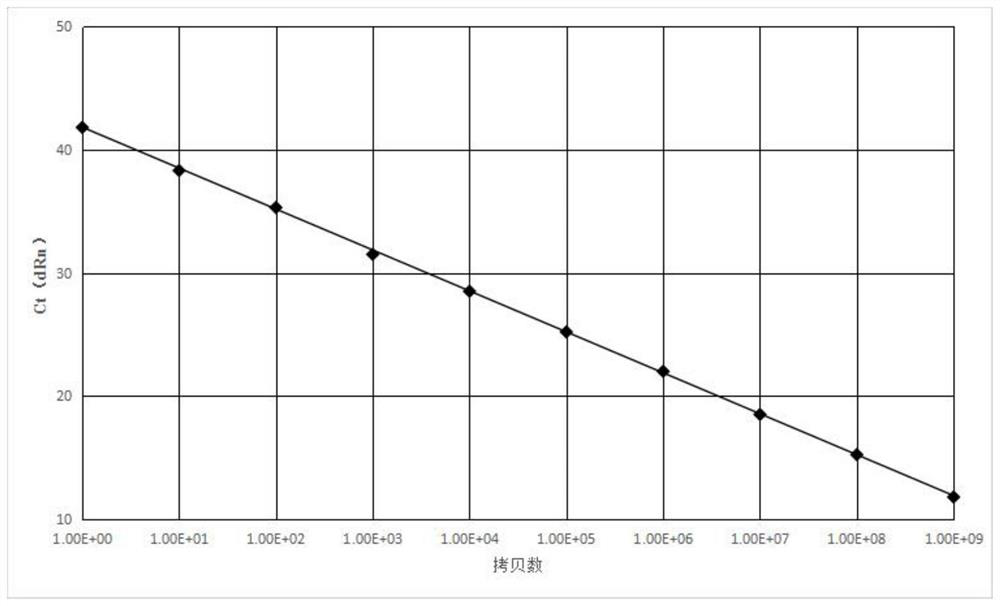
The present invention relates to a kind of mixed gene that is used for detecting fusion gene, and its gene sequence is as shown in SEQ ID NO:1, comprises the BCR-ABL fusion gene of SEQ ID NO:2, the AML-ETO fusion gene of SEQ ID NO:3 , the PML-RARA fusion gene of SEQ ID NO:4 and the ABL internal reference gene of SEQ ID NO:5; also relate to the standard plasmid that contains mixed gene, comprise the PUC57 plasmid of SEQ ID NO:6; Also relate to a kind of standard plasmid that contains The kit; also relates to a preparation method of a standard plasmid. Its advantage is, can be used for in the leukemia-associated fusion gene in quantitative detection people's bone marrow or peripheral blood sample BCR-ABL, RUNX1-RUNX1T1 (AML-ETO) and PML-RARA fusion gene double standard curve establishment; Effectively improve Improve detection efficiency and accuracy, reduce human error, and avoid multi-plasmid contamination; the minimum detection copy number of standard plasmids is 1*10 0 pcs / ml; easy to operate, not easy to pollute, large detection range, can effectively reduce detection cost.
Owner:上海科医联创医学检验所有限公司
Gastric cancer prognosis biomarker and application thereof
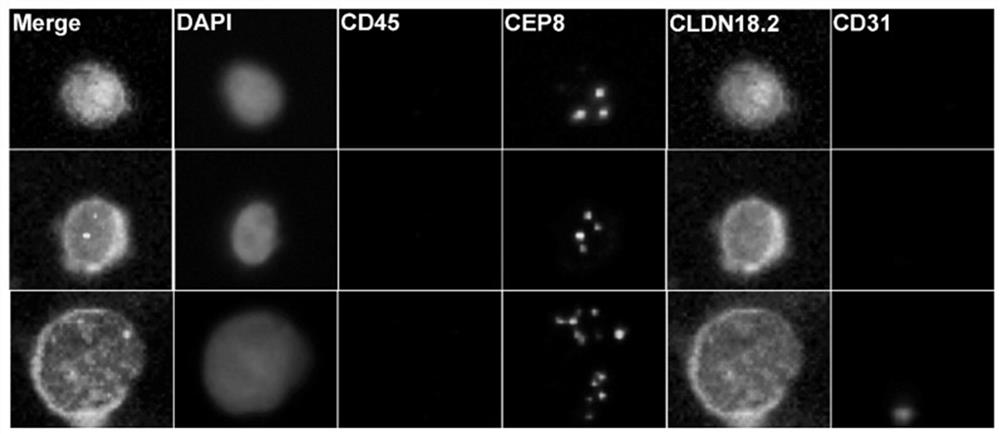
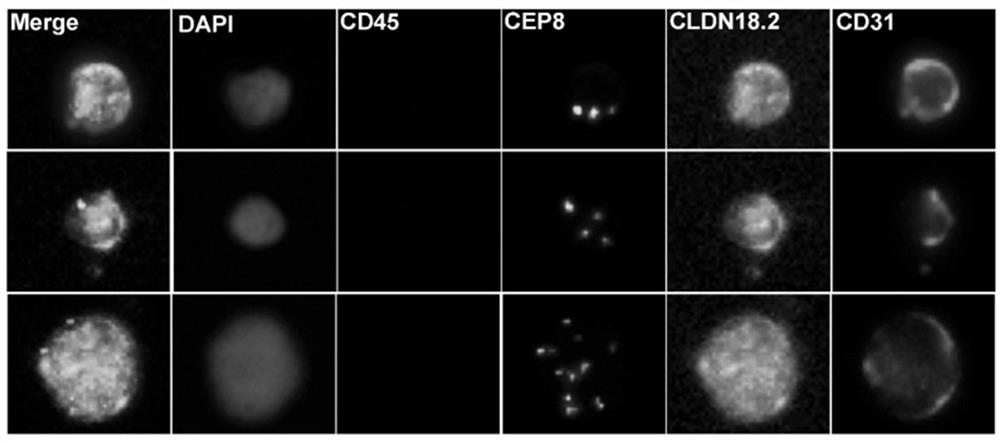
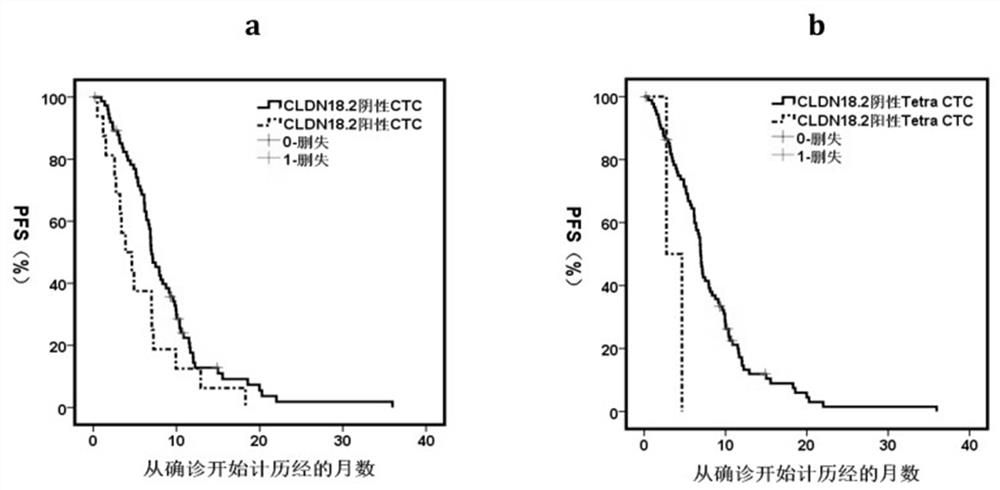
ActiveCN114428174AThe acquisition process is minimally invasive and safeDynamic MonitoringBiological material analysisBiological testingOncologyAdvanced gastric cancer
According to the invention, CLDN18.2 positive CTC detected in peripheral blood of a patient suffering from advanced gastric cancer or CLDN18.2 positive DTC detected in ascites of a patient suffering from peritoneal metastasis gastric cancer is disclosed for the first time, so that the patient is indicated to have shorter PFS and OS, the treatment effect and total survival of the patient suffering from advanced gastric cancer can be effectively predicted before treatment, only 6 mL of peripheral blood sample of the patient suffering from gastric cancer needs to be extracted during operation, and the operation is simple and convenient. Complex surgery and pathological material taking under an endoscope are not needed, the specimen obtaining process is more minimally invasive and safer, and the method has extremely high popularization value.
Owner:BEIJING CANCER HOSPITAL PEKING UNIV CANCER HOSPITAL
Construction method of systemic lupus erythematosus atlas model
ActiveCN105040111BReasonable designEfficient acquisitionMicrobiological testing/measurementLibrary creationHuman bodyPeripheral blood specimen
A method for constructing a systemic lupus erythematosus map model, comprising the following steps: setting a systemic lupus erythematosus group and a normal control group, each group including several peripheral blood samples from different human bodies that have been separated from the human body; The DNA in the blood sample is obtained to obtain the DNA test solution; the DNA content and integrity in the DNA test solution are measured, and if the test is qualified, then the next step is performed; multiplex PCR technology is used to construct a gene library and detect the gene library , if the test is qualified, then proceed to the next step; use high-throughput sequencing technology to sequence the gene library; analyze the sequencing results to construct a systemic lupus erythematosus map model. The construction method of the systemic lupus erythematosus atlas model mentioned above is reasonable and feasible in design, and can effectively obtain SLE-related information as an intermediate result.
Owner:中国人民解放军联勤保障部队第九二四医院
Kit for one-step real-time fluorescent quantitative reverse transcription polymerase chain reaction detection of human miR-1290
InactiveCN109536589AMicrobiological testing/measurementPeripheral blood specimenReverse transcription polymerase chain reaction
The invention provides a kit for real-time fluorescent quantitative RT-PCR detection of human miR-1290. The kit takes total mRNA as a template, so that reverse transcription and real-time fluorescentquantitative PCR can be carried out in a same reaction system, opening of a tube cap is not needed in the reaction process, cross-contamination is avoided, and the expression quantity of human miR-1290 in a sample can be accurately quantitatively detected. The kit can be used for detecting the expression of miR-1290 in peripheral blood samples of patients in clinical and scientific research, and can be used for assistant diagnosis, guide treatment, recurrence and prognosis reminding of colorectal cancer.
Owner:安徽普元生物科技股份有限公司
Method for differential diagnosis of EBV infected cell subtype and application thereof
PendingCN114214456AComplete formStrong specificityMicrobiological testing/measurementMicroorganism based processesInfected cellStaining
The invention belongs to the field of medical science, and particularly discloses a method for differential diagnosis of EBV infected cell subtypes and application thereof, and the method comprises the following steps: staining cells by using a fluorescence labeled lymphocyte specific antibody, specifically recognizing EBV infection characteristic RNA: EBERs in the cells by using a FISH probe, and then detecting EBV infected cell subgroups by flow cytometry, the cell subtype infected by the EBV is identified. The flow cytometry and the in-situ fluorescence hybridization are combined for use, a method capable of directly, quickly, conveniently and reliably detecting and identifying the EBV infected cell subtype of lymphocytes in a clinical peripheral blood sample is established, and the method has extremely high clinical application value.
Owner:CHILDRENS HOSPITAL OF CHONGQING MEDICAL UNIV +1
Mixed gene, standard plasmid and kit for detecting fusion gene as well as preparation method of standard plasmid
ActiveCN108676848AImprove detection efficiencyImprove detection accuracyMicrobiological testing/measurementDNA/RNA fragmentationPeripheral blood specimenBCR-ABL Fusion Gene
The invention relates to a mixed gene for a detecting fusion gene. A gene sequence of the mixed gene is shown in SEQ ID NO:1. The mixed gene comprises a BCR-ABL fusion gene shown in SEQ ID NO:2, an AML-ETO fusion gene shown in SEQ ID NO:3, a PML-RARA fusion gene shown in SEQ ID NO:4 and an ABL reference gene shown in SEQ ID NO: 5. The invention further relates to standard plasmid containing the mixed gene, and the standard plasmid comprises PUC57 plasmid shown in SEQ ID NO: 6. The invention further relates to a kit containing the standard plasmid and further relates to a preparation method ofthe standard plasmid. The mixed gene has the advantages that the mixed gene can be applied to establishing a double-standard curve which is used for quantitatively detecting BCR-ABL, RUNX1-RUNX1T1 (AML-ETO) and PML-RARA fusion gene in a leukemia related fusion gene in human marrow or a peripheral blood sample; detection efficiency and accuracy are effectively improved, manual operation errors arereduced, and multi-plasmid pollution is prevented from happening; the lowest detection copy number of the standard plasmid is 1*10<0> per ml; operation is simple, pollution is not prone to happening,the detection range is large, and detection cost can be effectively reduced.
Owner:上海科医联创医学检验所有限公司
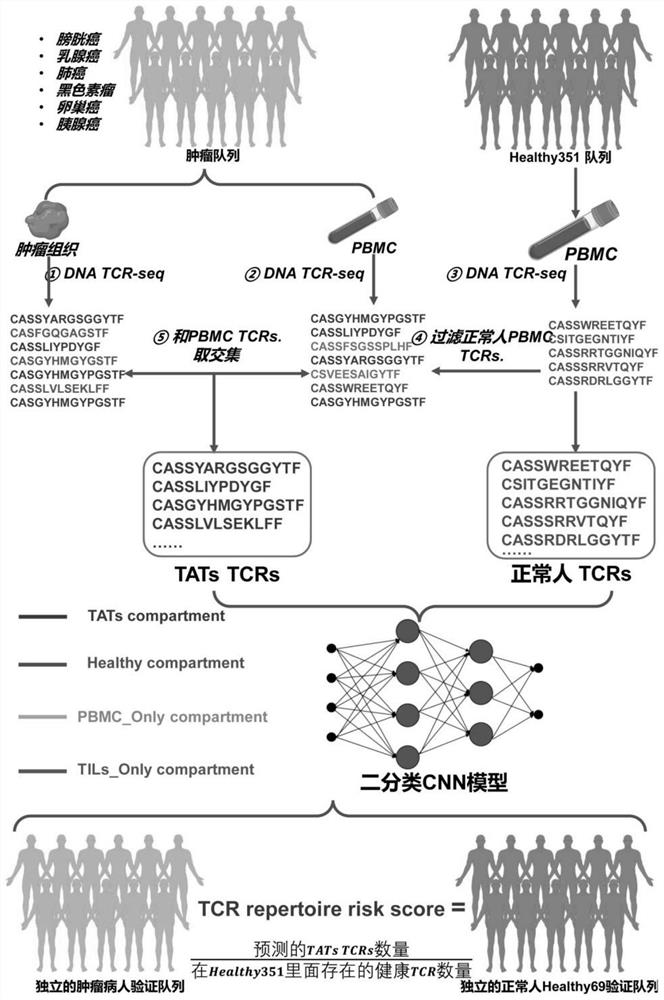
Method for determining TAT cells
PendingCN114591909AMicrobiological testing/measurementBlood/immune system cellsCancer cellBiopsy methods
The invention provides a method for determining TAT cells. Compared with non-cancer related T cells, the TAT cell is expected to have higher specific binding capacity with cancer cells and higher capacity of killing the cancer cells. Meanwhile, the TAT-TCR sequence contained in the TAT cell can also be used or combined with transcriptome information for cancer screening, and the method is high in sensitivity and specificity. Only the peripheral blood sample of a subject needs to be collected, the invasive property is small, more information can be utilized, an existing tumor liquid biopsy method can be well supplemented, early-stage asymptomatic tumors are potentially detected, cancer diagnosis and treatment are integrated, and important application value is achieved.
Owner:TSINGHUA UNIV
Plasmid standard product for detecting HLA-DP gene rs3077 locus polymorphism and preparation method thereof
InactiveCN105950718AGuaranteed accuracyGuaranteed reliabilityMicrobiological testing/measurementDNA/RNA fragmentationEthylene diaminePeripheral blood specimen
The invention provides a plasmid standard product for detecting HLA-DP gene rs3077 locus polymorphism. A preparation method of the plasmid standard product comprises the following steps of (1) designing primers of an HLA-DP gene rs3077 locus, wherein the sequences of forward and reverse primers are respectively 5'-AATAACTGTGTGTGTTGCTG-3' and 5'-TCAATATCCTCAGACCTTTC-3'; (2) selecting EDTA (Ethylene Diamine Tetraacetic Acid) anti-coagulation peripheral blood specimens of the CC genotype and TT genotype of the HLA-DP gene rs3077 locus; and (3) building plasmid standard products including three different genotype target fragments of the HLA-DP gene rs3077 locus. The standard product has the advantages that the purity is high; the cost is low; the storage is easy; the accuracy is high; the specificity and the stability are good; and experiment errors can be reduced, and the like. The plasmid standard product can be used for HLA-DP gene rs3077 locus polymorphism detection for a long time.
Owner:CHENGDU ZHONGCHUANG QINGKE MEDICAL LAB CO LTD
Method for treating complementary determining region (CDR) of T cell receptor (TCR) beta chain
InactiveCN107345242ACan understand the immune statusReasonable designMicrobiological testing/measurementDiseaseComplementarity determining region
The invention relates to a method for treating a complementary determining region (CDR) of a T cell receptor (TCR) beta chain. The method comprises the following steps: setting an experimental group and a control group, wherein each group comprises a plurality of parts of peripheral blood samples which are dissimilar to a human body and get away from the human body; separating lymphocytes of the peripheral blood samples, and respectively extracting deoxyribonucleic acid (DNA) in the lymphocytes of the peripheral blood samples; adopting a multiplex polymerase chain reaction (PCR) technique to establish a DNA library; carrying out high-throughput sequencing on the DNA library, and carrying out data analysis on the CDRs of TCR beta chains in the experimental group and the control group. The method for treating the CDR of the TCR beta chain is reasonable and feasible in design, and can be used for comprehensively analyzing the dynamic change characteristics of a receptor repertoire of the CDRs of the TCR beta chains in bodies of patients with hepatitis B virus-related cirrhosis at the decompensatory stage before and after transplantation and understanding the immune status of the patients so as to guide medication during monitoring disease situation after transplantation, prevent postoperative rejection and infection and provide a basis for the pathogenesis of diseases.
Owner:眭维国 +1
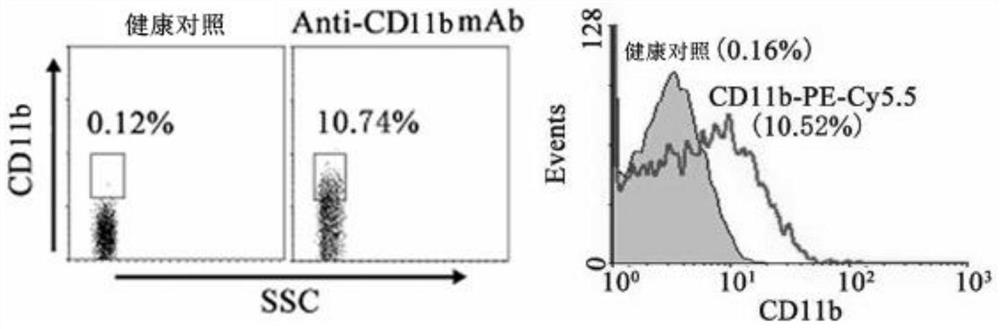
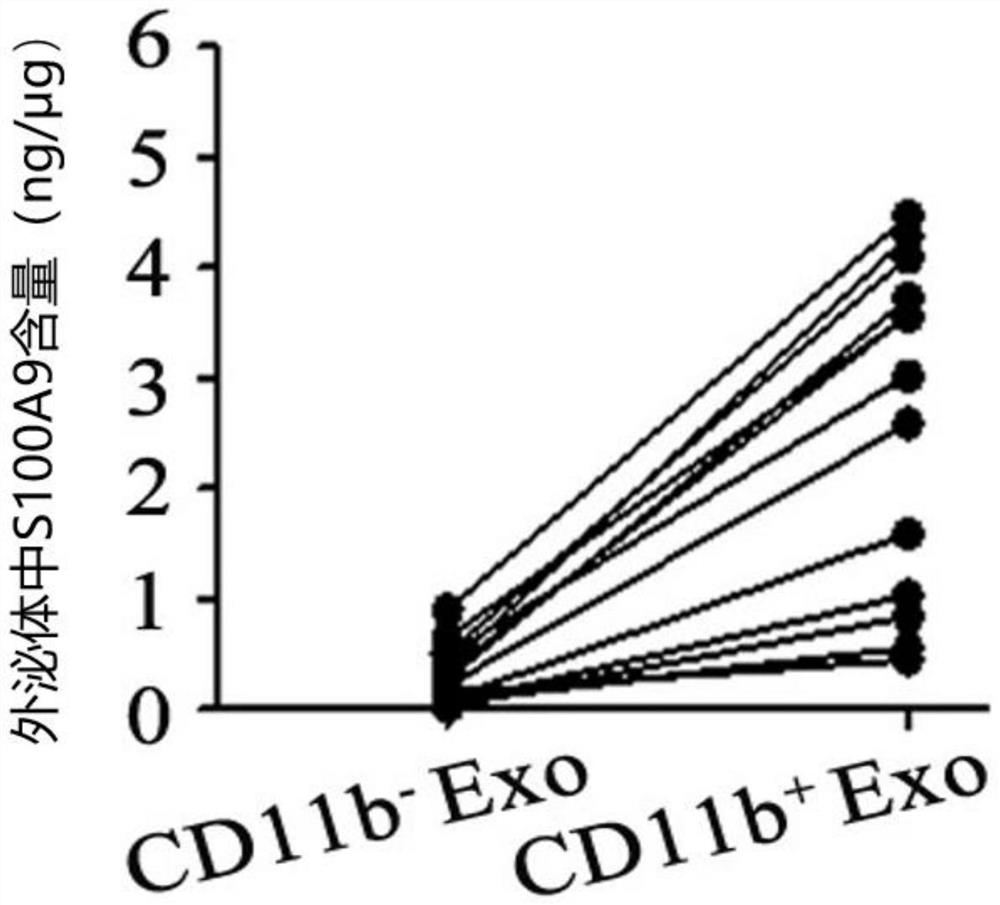
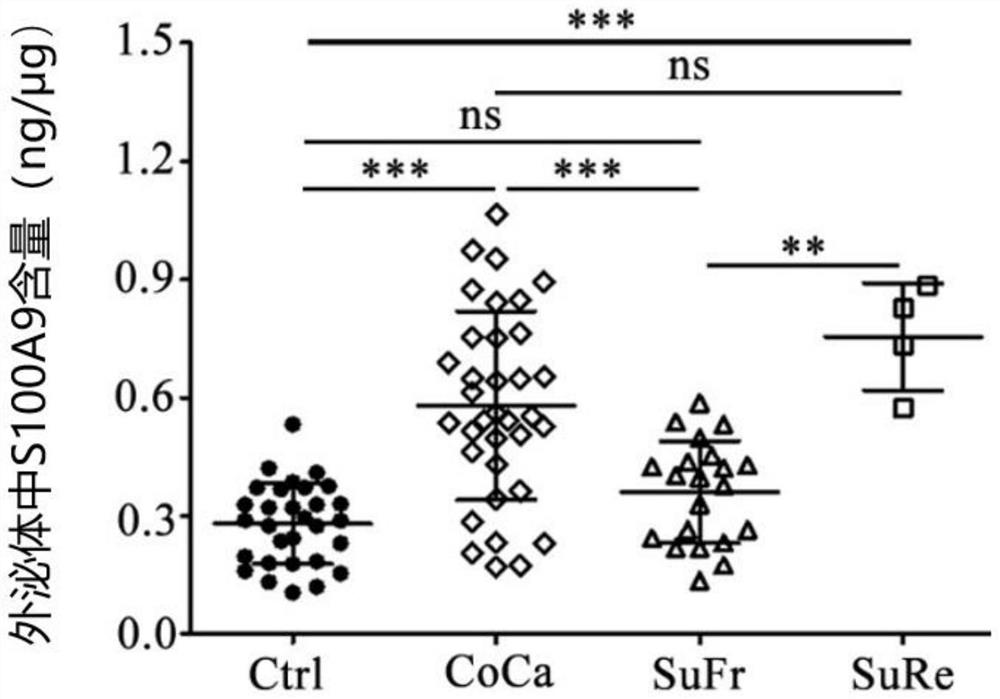
Application of CD11b positive exosome in tumor prognosis evaluation
PendingCN113514385AReduce mortalityHigh expressionIndividual particle analysisPeripheral blood specimenExosome
The invention belongs to the technical field of biological medicines, and particularly relates to an application of a CD11b positive exosome in tumor prognosis evaluation. According to the invention, peripheral blood samples of healthy people, colorectal cancer patients and patients before and after an operation are collected, human plasma CD11b + exosomes and CD11b-exosomes are sorted by adopting flow cytometry, and the content of S100A9 in the CD11b + exosomes is detected by utilizing ELISA. The S100A9 protein in the plasma CD11b + exosomes is determined to have a significant high expression quantity, and has sensitivity and specificity for judging recurrence and metastasis of a colorectal cancer; and meanwhile, the application has the characteristics of simple operation, sensitive detection, good specificity, high repeatability and the like. The application confirms that the expression level of the S100A9 protein in CD11b + exosomes is related to the prognosis of the postoperative colorectal cancer patient, can be used for judging the prognosis of the colorectal cancer patient, and has important guiding significance for reducing the death rate of the colorectal cancer and improving the prognosis.
Owner:JIANGSU UNIV
Method for detecting multi-site mutation of free DNA of lung tumor plasma
PendingCN113999906AHigh sensitivityImprove featuresMicrobiological testing/measurementDNA/RNA fragmentationPeripheral blood specimenLung tumor
The invention provides a method for detectingmulti-site mutation of free DNA of lung tumor plasma. The method is characterized in that a specific primer, a specific PNA probe and a capture technology are adopted to improve ctDNA detection and specificity, so that trace ctDNA can be detected. The invention provides primers, probes and a detection method for gene variation detection in a peripheral blood sample of a lung space-occupying lesion patient. The method has the advantages of high sensitivity, strong specificity, good repeatability, simplicity in operation, rapidness in detection, safety and the like, and has an auxiliary effect on diagnosis of lung cancer.
Owner:深圳思凝一云科技有限公司
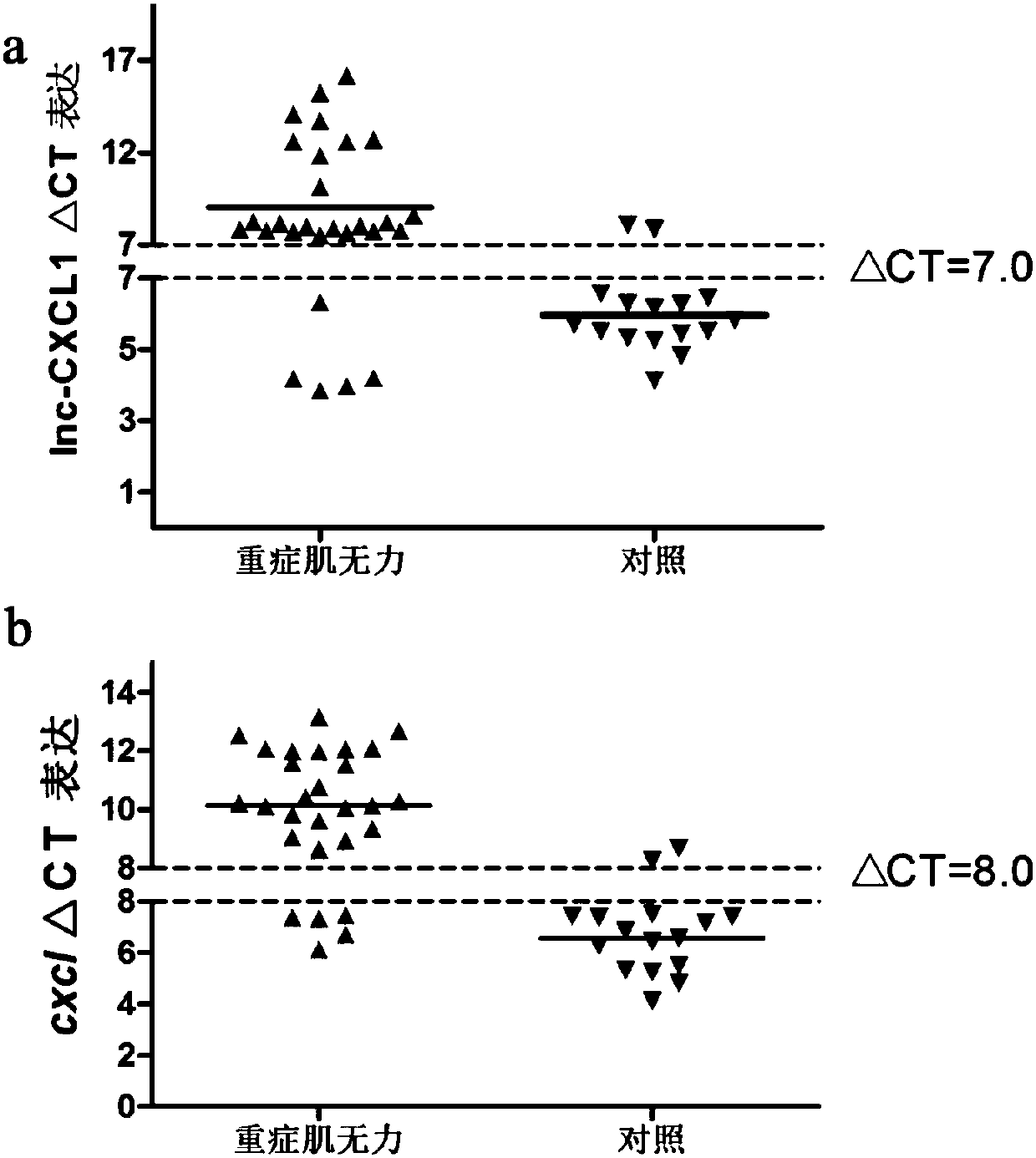
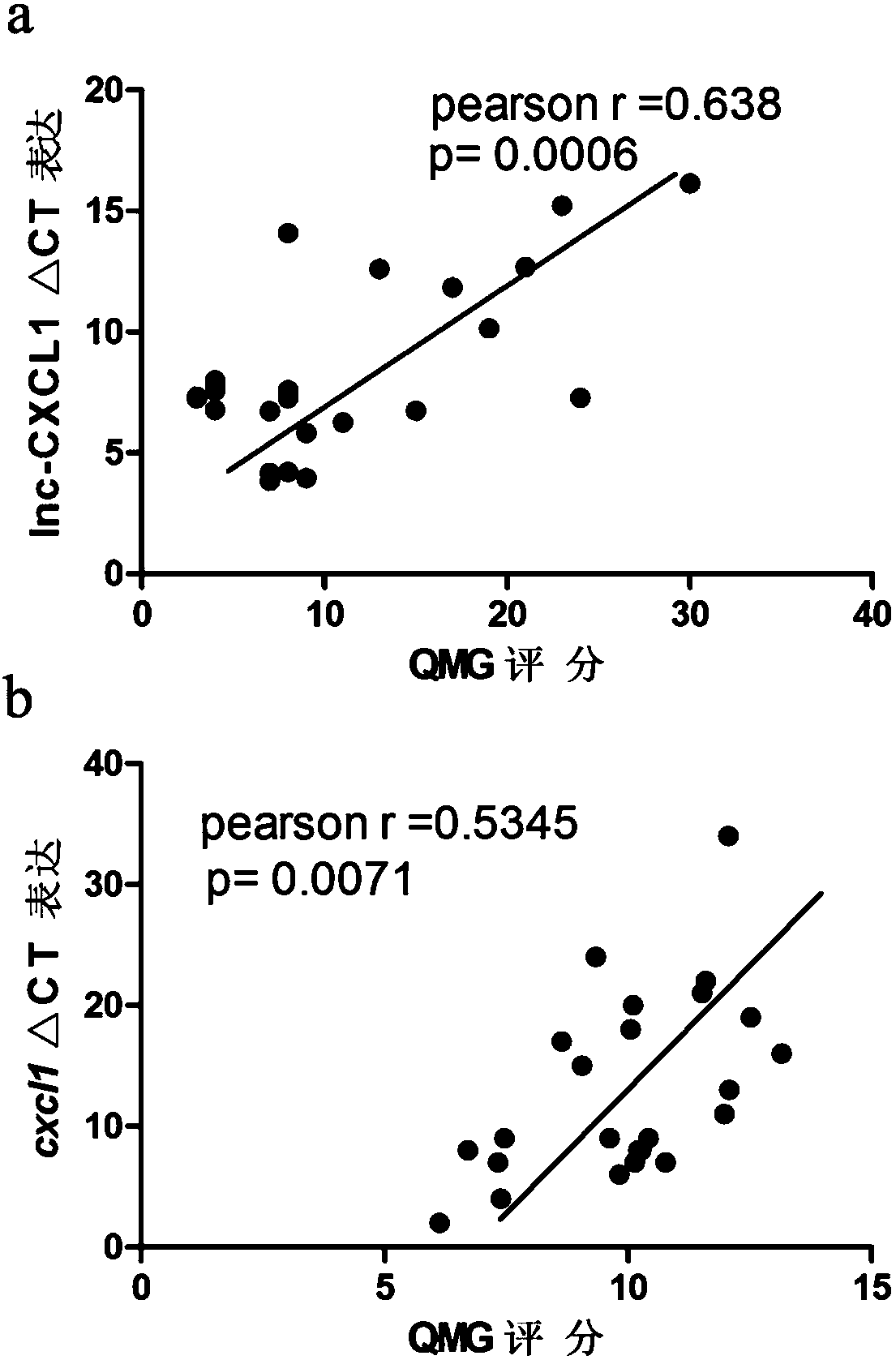
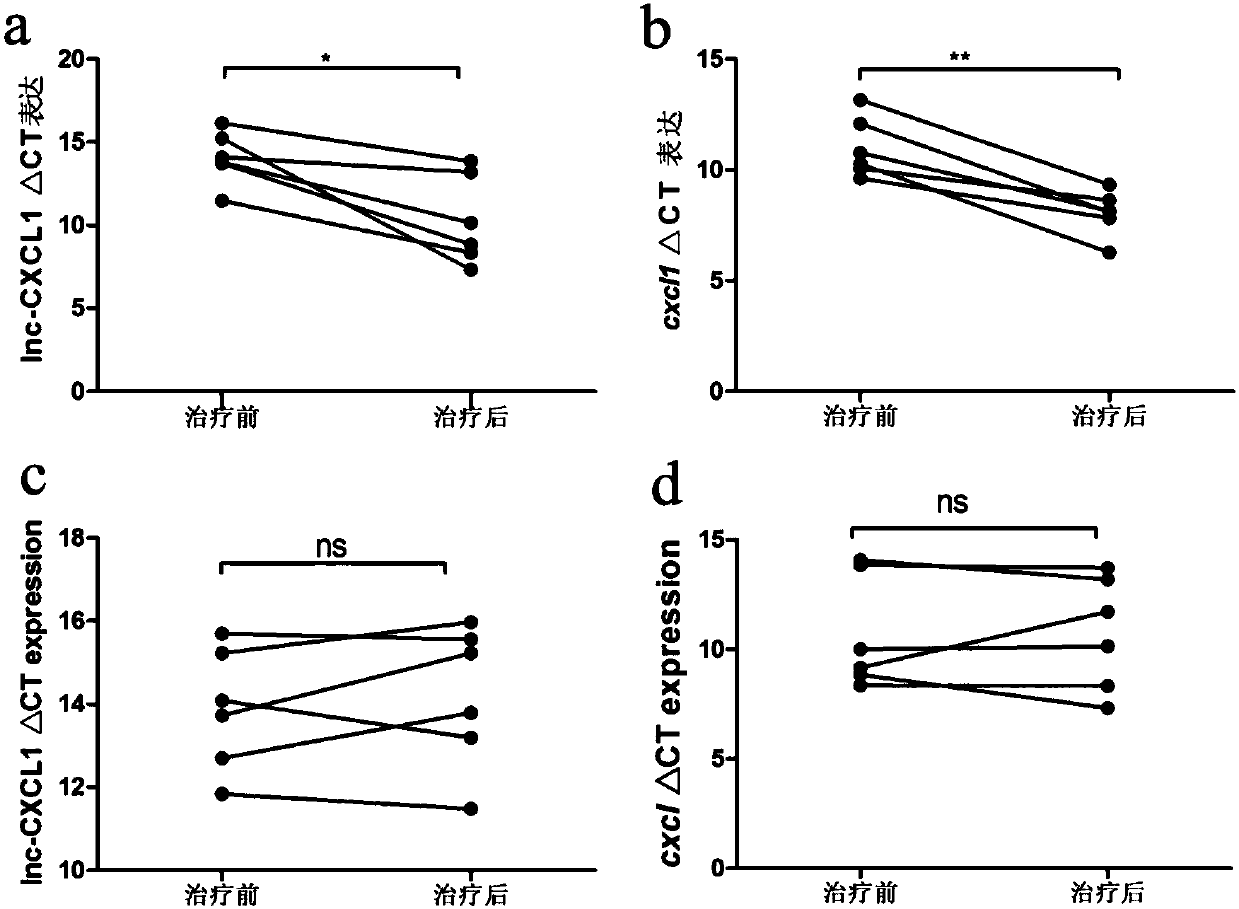
A myasthenia gravis detection kit and application using a combination of non-coding lnc-cxcl1 and coding gene cxcl1 as a detection or diagnostic screening marker
ActiveCN106119348BMicrobiological testing/measurementDNA/RNA fragmentationMyasthenia gravisPeripheral blood specimen
The invention discloses a myasthenia gravis detection reagent kit with the combination of non-coded lnc-CXCL1 and a coded gene cxcl1 as a detection or diagnosis screening marker and application. By detecting the expression levels of lnc-CXCL1 and the coded gene cxcl1 in peripheral blood specimens of patients suffering from myasthenia gravis in a relative quantification mode, the reagent kit achieves the effects of screening patients suffering from myasthenia gravis, carrying out QMG on clinical symptoms, and judging hormone therapy.
Owner:XIANGYA HOSPITAL CENT SOUTH UNIV
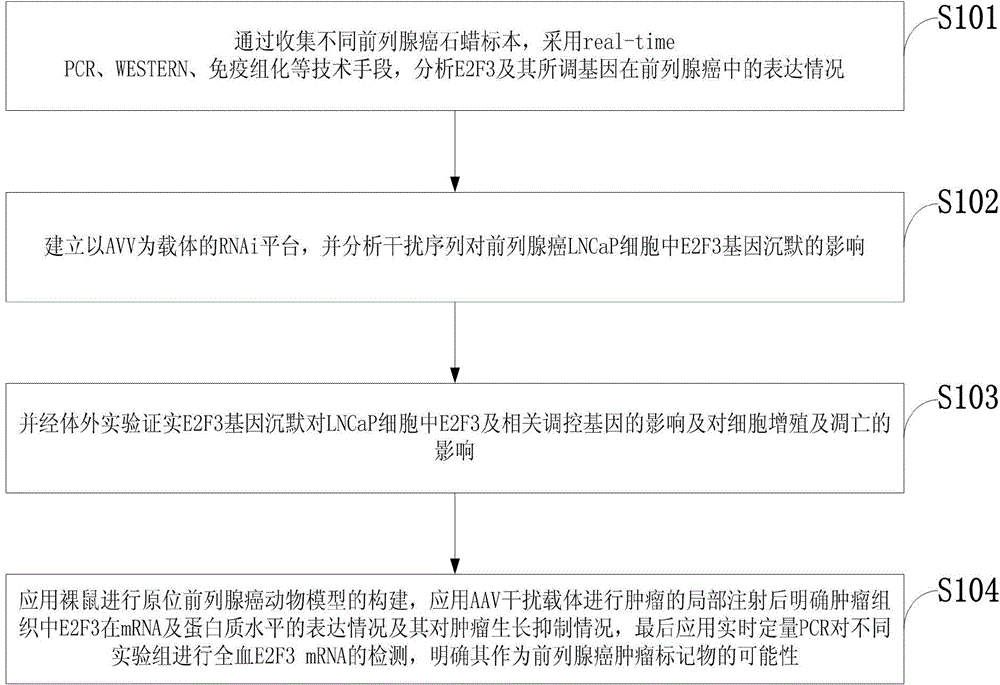
Method for analyzing influences of E2F3 gene slicing on whole blood E2F3 in prostate cancer animal model
InactiveCN104131088APrevent proliferationGrowth inhibitionMicrobiological testing/measurementBiological material analysisApoptosisProstate cancer
The invention discloses a method for analyzing influences of E2F3 gene silencing on E2F3 gene slicing on whole blood E2F3 in a prostate cancer animal model. The method comprises the following steps: clarifying a relationship between E2F3 and each of grading, staging and prognosis of the prostate cancer through immunohistochemical study of the expression of E2F3 in different prostate cancer tissues; silencing E2F3 gene by constructing an AAV virus vector for E2F3, and carrying out related in-vitro function experiment; and successfully applying the AAV virus vector by establishing a nude mouse prostate cancer model to carry out in vivo experiment, and clarifying the E2F3 mRNA expression in the nude mouse whole blood before and after the E2F3 gene silencing. The foundation is laid for subsequent prostate cancer gene therapy in vivo experiment with E2F3 as a target by adopting the E2F3 gene as an efficient target for prostate cancer gene therapy in the invention; and a recombinant RNA interference AAV virus for E2F3 can inhibit the proliferation of tumor cells, can promote cell apoptosis, effectively inhibits the tumor growth of the animal model, reduces the expression of E2F3 in peripheral blood specimens, and provides the reliable theoretical and experimental foundation for tumor gene silencing therapy with the E2F3 as the target.
Owner:天津市泌尿外科研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com