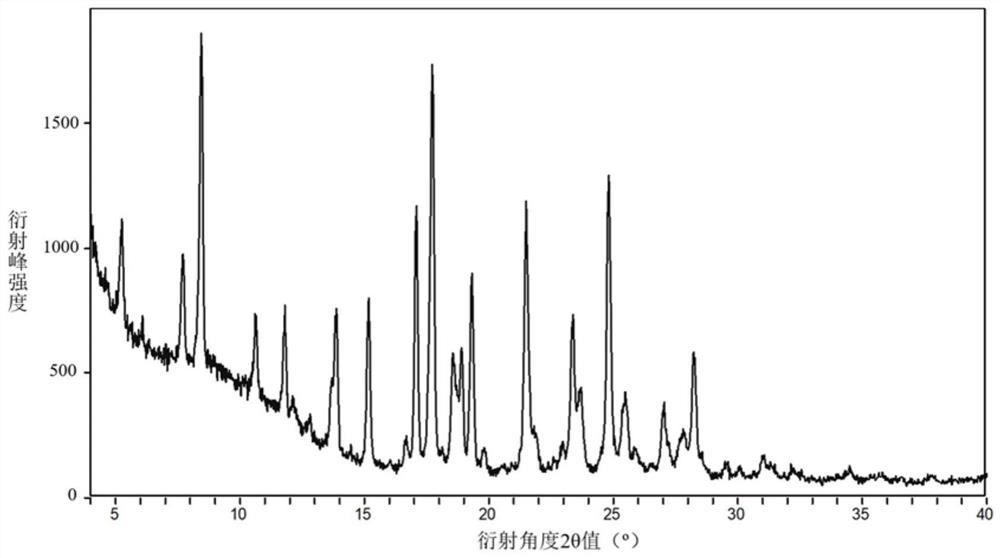
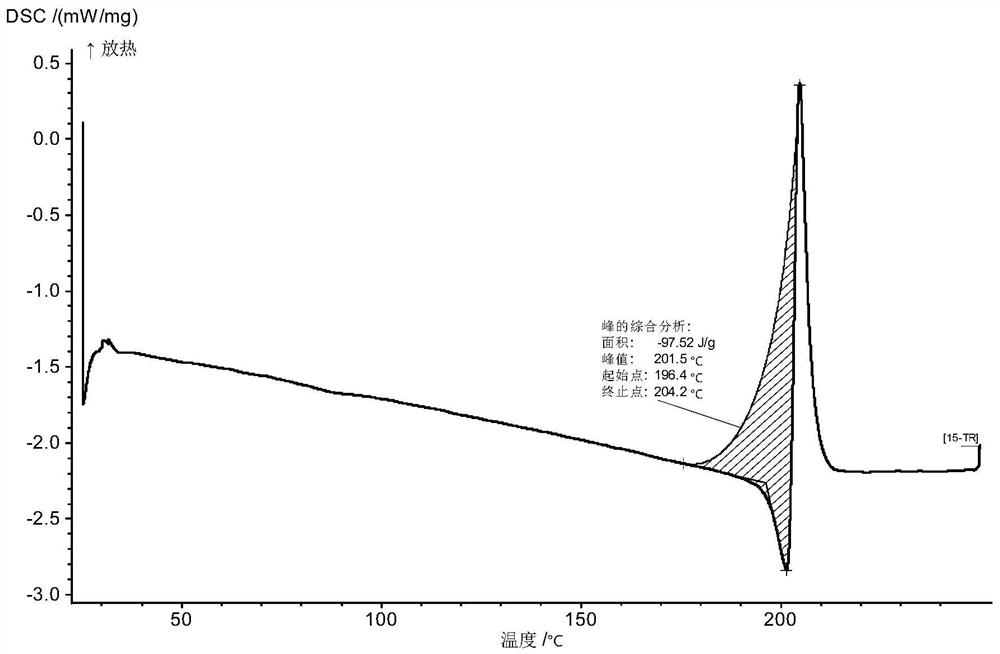
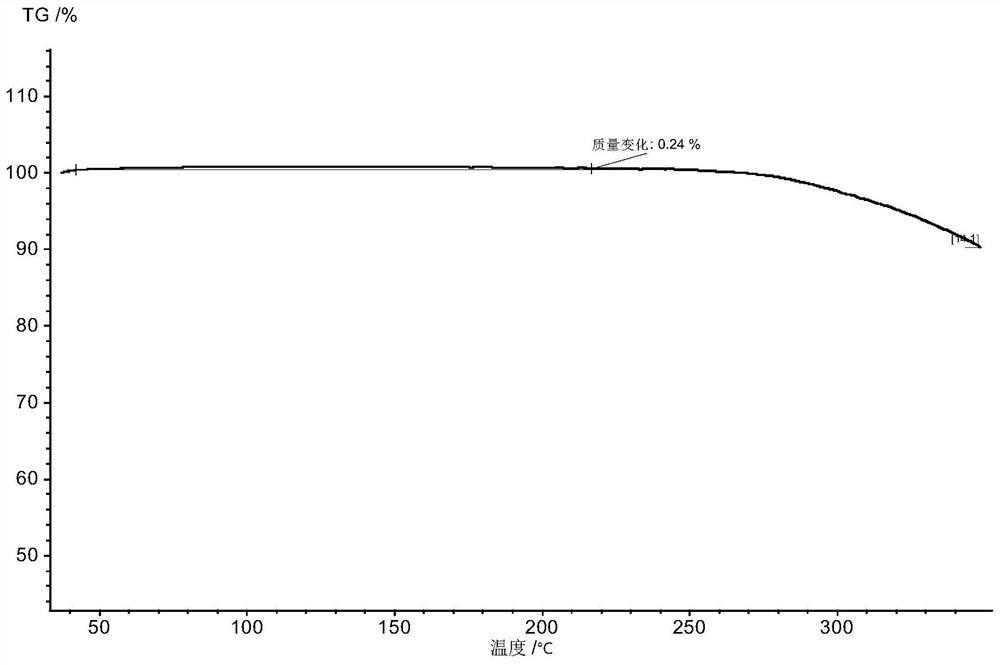
Inhibitor salt or crystal form containing di-fused ring derivative and preparation method and application of inhibitor salt or crystal form
A crystal form and diazabicyclo technology, applied in the field of drug synthesis, can solve the problems of large clinical needs and no specific targeted drugs for RET targets
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0200]Unless stated to the contrary, the terms used in the specification and claims have the following meanings.
[0201] The term "alkyl" refers to a saturated aliphatic hydrocarbon group which is a straight or branched chain group containing 1 to 20 carbon atoms, preferably an alkyl group containing 1 to 8 carbon atoms, more preferably 1 to 6 carbon atoms An alkyl group, most preferably an alkyl group of 1 to 3 carbon atoms. Non-limiting examples include methyl, ethyl, n-propyl, isopropyl, n-butyl, isobutyl, tert-butyl, sec-butyl, n-pentyl, 1,1-dimethylpropyl, 1 ,2-Dimethylpropyl, 2,2-Dimethylpropyl, 1-ethylpropyl, 2-methylbutyl, 3-methylbutyl, n-hexyl, 1-ethyl-2- Methylpropyl, 1,1,2-trimethylpropyl, 1,1-dimethylbutyl, 1,2-dimethylbutyl, 2,2-dimethylbutyl, 1,3 -Dimethylbutyl, 2-ethylbutyl, 2-methylpentyl, 3-methylpentyl, 4-methylpentyl, 2,3-dimethylbutyl, n-heptyl, 2 -Methylhexyl, 3-methylhexyl, 4-methylhexyl, 5-methylhexyl, 2,3-dimethylpentyl, 2,4-dimethylpentyl, 2,2-dim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com