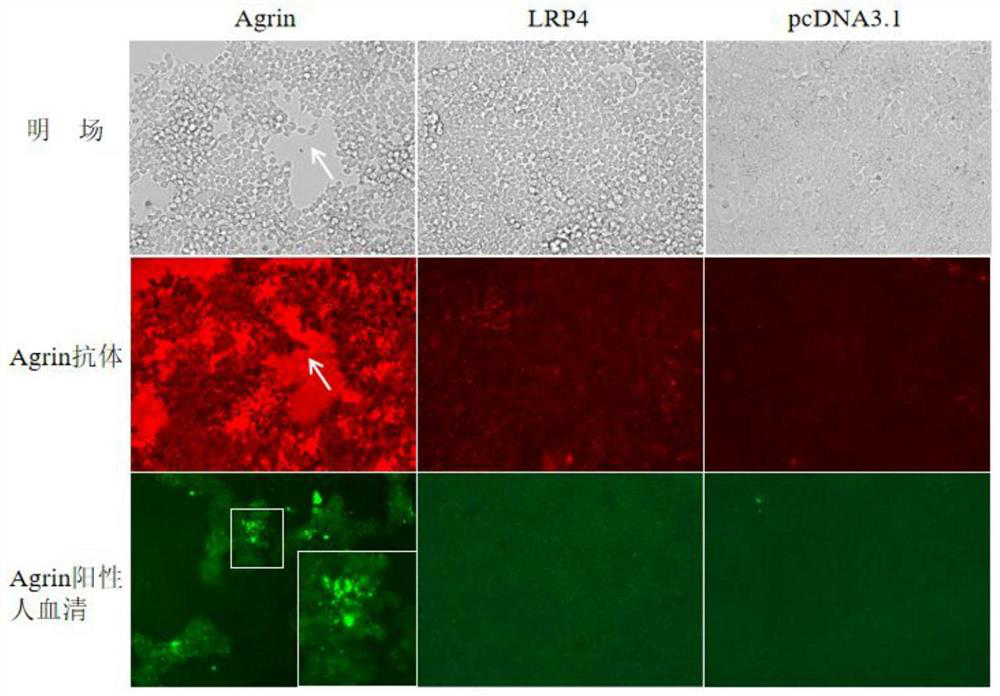
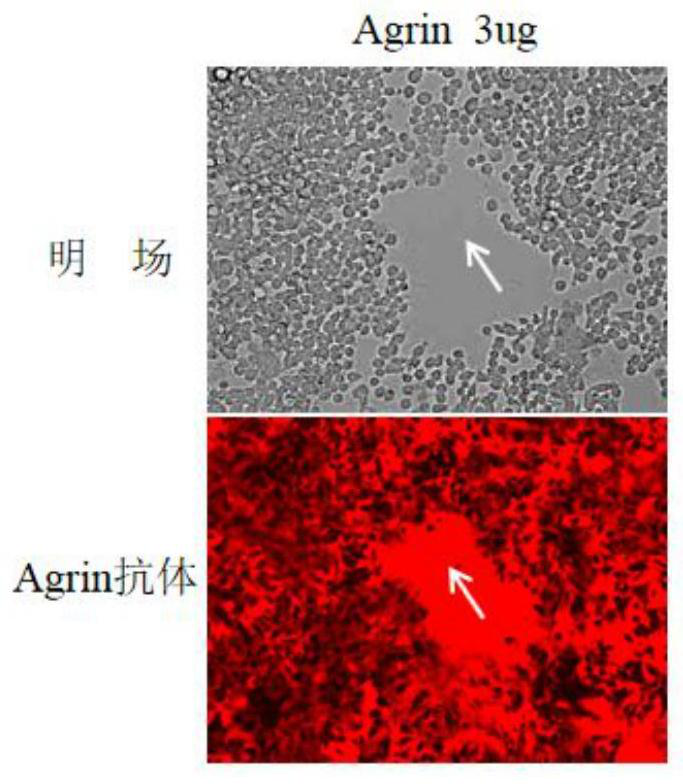
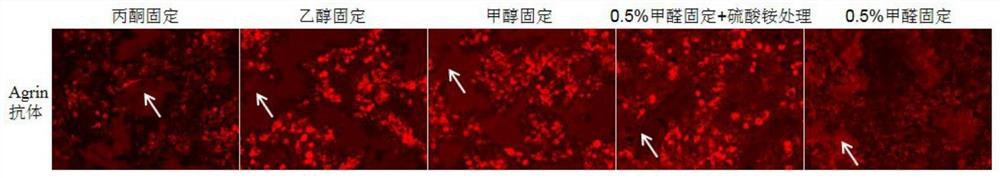
Reagent and method for improving quality of Agrin protein immunofluorescence flaking and application
An immunofluorescence and reagent technology, applied in the field of reagents to improve the quality of Agrin protein immunofluorescence production, can solve the problems of poor growth of Agrin protein crawling cells, poor live cell immunofluorescence signal, etc., and achieve the enhancement of live cell immunofluorescence signal, Improve poor quality and reduce secretion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Agrin affects cell migration / proliferation
[0044] Step 1. Agrin expression plasmid vector construction
[0045] 1. Gene synthesis
[0046] The Agrin gene sequence was obtained from the GenBank sequence database, and the gene sequence was synthesized.
[0047] 2. Vector construction
[0048] The target gene Agrin was connected to the pcDNA3.1 vector, and the enzyme cutting sites were selected to be NheI and XbaI. After the sequencing was correct, the plasmid was extracted for subsequent transfection. The specific construction steps are as follows:
[0049] 2.1. Using the plasmid containing the target gene Agrin (6207bp) as a template, the upstream primer Agrin-NheI-F: atccGCTAGCGCCGCCACCatggccggccggtcccacccggg (SEQ ID NO.5), the downstream primer Agrin-XbaI-R: TGCTCTAGATCATGGGGTGGGGCAGGGCCGC (SEQ ID NO.6), PCR Amplified the Agrin gene;
[0050] PCR amplification system (50μl): template 50ng, Agrin-NheI-F (10μM) 1μl, Agrin-XbaI-R (10μM) 1μl, FastPfu DNA Polymerase ...
Embodiment 2
[0073] Detection of Agrin cell proliferation
[0074] Add small slides to a 10cm cell culture dish, after PDL treatment, inoculate cells, culture overnight, transfect Agrin expression plasmid (6ug) and control pcDNA3.1 (6ug) the next day, transfection steps are the same as in Example 1, without Special limited. After 48 hours of transfection, the small slides were taken out and placed in a 96-well plate, and the cell proliferation was measured with a CCK-8 kit (purchased from Beyotime Biotechnology Company), and three groups of repeated experiments were set up.
[0075] Experimental results such as Figure 4 As shown, compared with the control group, the proliferation of cells transfected with Agrin was significantly reduced. That Agrin affects the growth of cells.
Embodiment 3
[0077] Validation of the Agrin protein signal secreted into the cell supernatant
[0078] The Agrin expression plasmid and the control pcDNA3.1 were transfected, and the cell supernatants were collected after 24 hours of culture, and then added to DMEM medium to continue culture for 96 hours, and the supernatants were collected respectively. Centrifuge the collected cell supernatant at 1000 rpm for 5 min, discard the precipitate, spot the centrifuged supernatant on the NC membrane, dry at 37°C, and verify the signal by immunospotting. The specific steps are as follows:
[0079] 1. Place the dried NC membrane in a clean 12-well plate, with the sample side facing up. Dilute the commercial Agrin antibody at 1:1000, add 200ul diluted antibody to the well with NC membrane, and incubate at room temperature for 30min;
[0080] 2. Wash with the working solution 3 times, 5 minutes each time;
[0081] 3. Incubate alkaline phosphatase (AP)-labeled goat anti-rabbit secondary antibody fo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com