Medicine for treating doxorubicin-induced cardiotoxic injury and application
A technology of cardiotoxicity and doxorubicin, applied in the field of biomedicine, can solve the problems of increasing the four-year cumulative incidence of the second malignant tumor, side effects, thrombocytopenia, etc., and achieve the effect of improving cardiomyocyte apoptosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
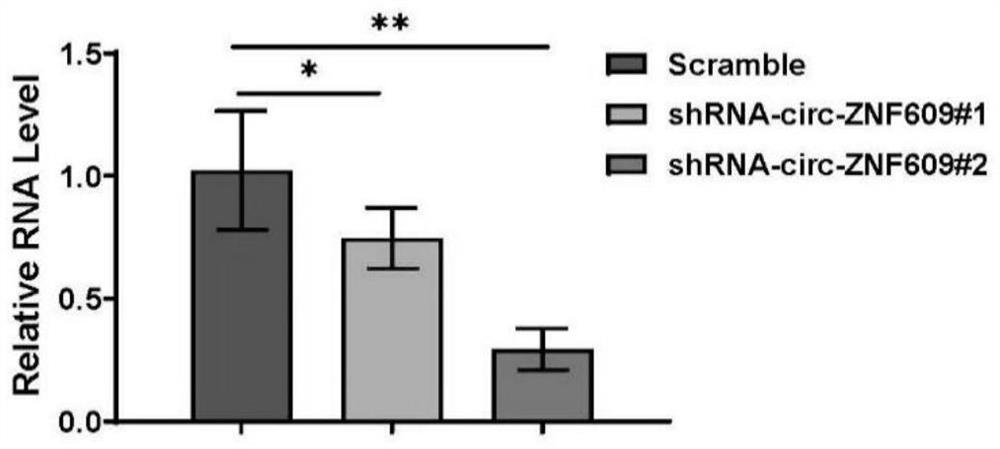
Embodiment 1
[0030] 1. Construction of circ-ZNF609 shRNA:
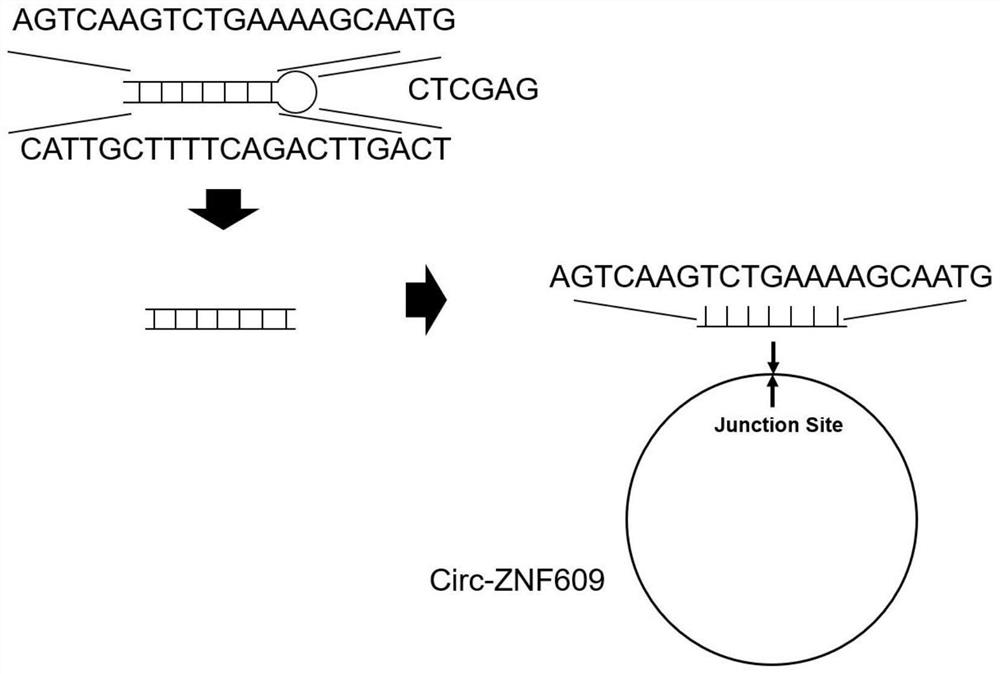
[0031] Firstly, the sequence of circZNF609 was obtained from the circBase database (species human source: hsa_circ_0000615 and mouse source: mmu_circ_0001797). Design siRNA sequences across the back-splicing site of the circular RNA:
[0032] siRNA#1 (SEQ ID NO.5): 5'-AGTCAAGTCTGAAAAGCAATGAT-3'
[0033] siRNA#2 (SEQ ID NO.4): 5'-AGTCAAGTCTGAAAAGCAATG-3'
[0034] After designing the siRNA, insert the shRNA structure template:
[0035] For shRNA#1:
[0036] Forward primer (SEQ ID NO.6): 5'-GATCAGTCAAGTCTGAAAAGCAATGATCTCGAGATCATTGCTTTTCAGACTTGACT TTTTTG-3'
[0037] Reverse primer (SEQ ID NO.7): 5'-AATT CAAAAAGTCAAGTCTGAAAAGCAATGATCTCGAGATCATTGCTTTTCAGACTTGACT-3'
[0038] For shRNA#2:
[0039] Forward primer (SEQ ID NO.2): 5'-GATCAGTCAAGTCTGAAAAGCAATGCTCGAGCATTGCTTTTCAGACTTGACT TTTTTG-3'
[0040] Reverse primer (SEQ ID NO.3): 5'-AATT CAAAAAAGTCAAGTCTGAAAAGCAATGCTCGAGCATTGCTTTTCAGACTTGACT-3';
[0041] The resulting shRNA sequen...
Embodiment 2
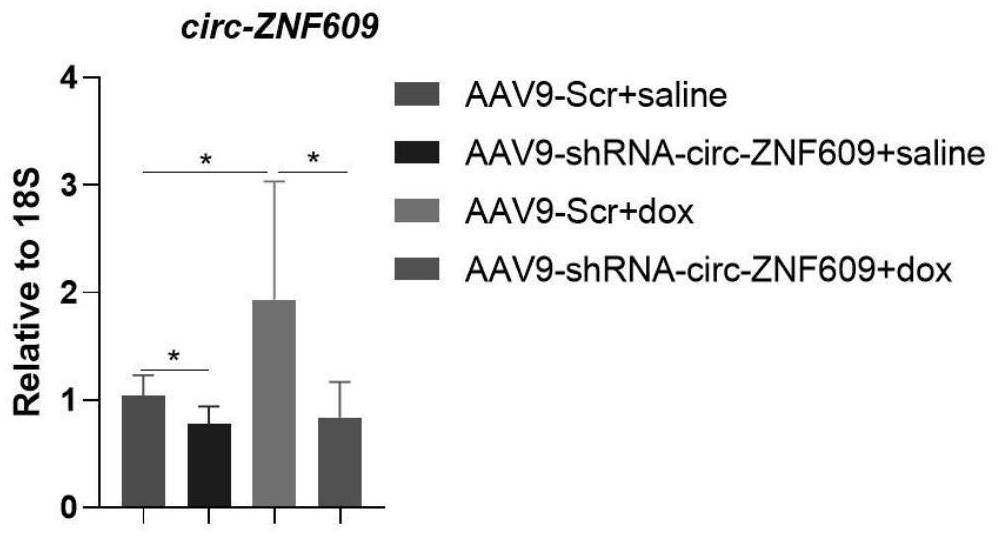
[0060] 1. Establishment of doxorubicin-induced cardiotoxic injury model in mice
[0061] Take C57BL / 6J wild-type male mice aged 8-10 weeks, and give the mice intraperitoneal injection of doxorubicin at a dose of 5 mg / kg to induce cardiotoxic injury to the mice, once a week for 4 weeks, and the corresponding model The control model, that is, the control group mice, were replaced by intraperitoneal injection of an equal volume of normal saline once a week for 4 weeks. The mice were sacrificed 7 days after the last administration was completed.
[0062] 2. AAV9-sh-circ-ZNF609 injection
[0063] will be 10 11 A dose of vg / mouse was injected into the mice through tail vein injection, and a cardiotoxic injury model was constructed 1 week later. Where vg represents vector genome. The specific experimental process is as follows:
[0064] First, divide the experimental mice into 4 groups, namely control virus + normal saline group, control virus + doxorubicin group, AAV9-shRNA-cir...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Virus titer | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com