Medicinal composition for treating ulcerative colitis and preparation method thereof
A technology for positioning drugs and colons, which can be used in drug combinations, pharmaceutical formulations, medical preparations with inactive ingredients, etc. It can solve the problems of complex raw materials and components, and achieve the effects of convenient use, prevention of recurrence, and improvement of diarrhea.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1 Preparation of the drug of the present invention
[0038] 1. Preparation of astragalus gastric drug release unit:
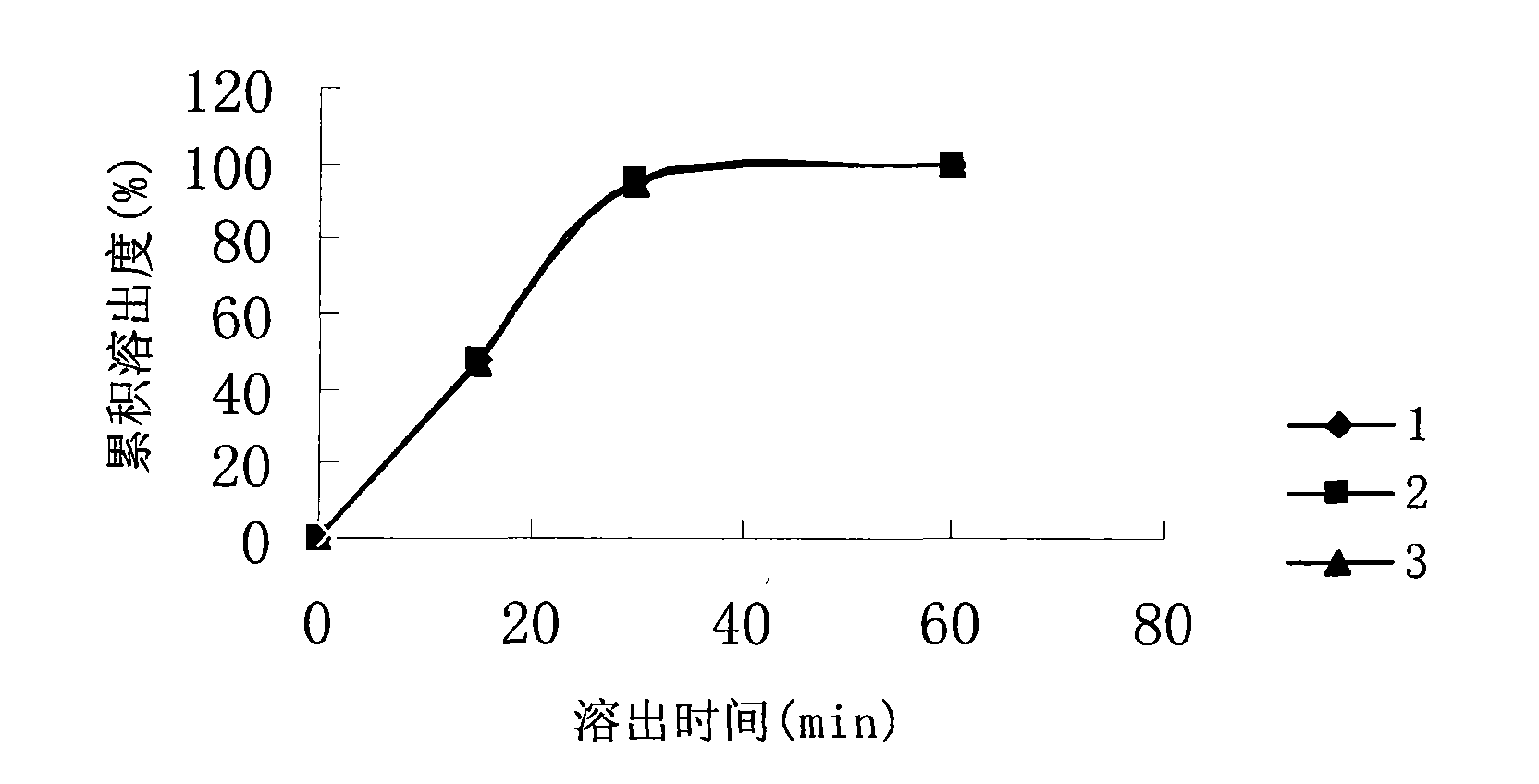
[0039] Take 2.5g of astragalus saponin extract, 7.5g of astragalus polysaccharide extract, 18g of microcrystalline cellulose, 3.5g of micropowder silica gel, 2g of sodium carboxymethyl starch, powder, powder, and mix well. A soft material is prepared with an appropriate amount of 50% ethanol as a wetting agent, extruded, and rounded to obtain pellets. Eudragit E100 alcohol-soluble coating is used, and the coating prescription is Eudragit E100 10g; talc 5g; PEG6000 1g; water 2g; ethanol 140g. Dissolve Eudragit E100 in about 70g ethanol; dissolve PEG6000 in water; add talc powder to the remaining ethanol and homogenize at high speed for 20 minutes; add PEG6000 solution and talc powder suspension to Eudragit E100 solution and stir evenly to obtain coating liquid. Adopt fluidized bed coating technology to coat the pellets to increase the weight by 5%. ...
Embodiment 2
[0050] Example 2 Preparation of the drug of the present invention
[0051] 1. Prescription of gastric drug release unit preparations
[0052] Astragalus Coated Pellets
[0053] Astragalus polysaccharide extract 398mg (contains polysaccharide 355mg) Astragalus saponin extract 136mg (contains saponins 100mg)
[0054] Microcrystalline cellulose 961mg Micropowder silica gel 187mg
[0055] Sodium Carboxymethyl Starch 107mg Coating 90mg
[0056]
[0057] 1879mg total
[0058] 2. Prescription of colon-specific drug release unit formulations
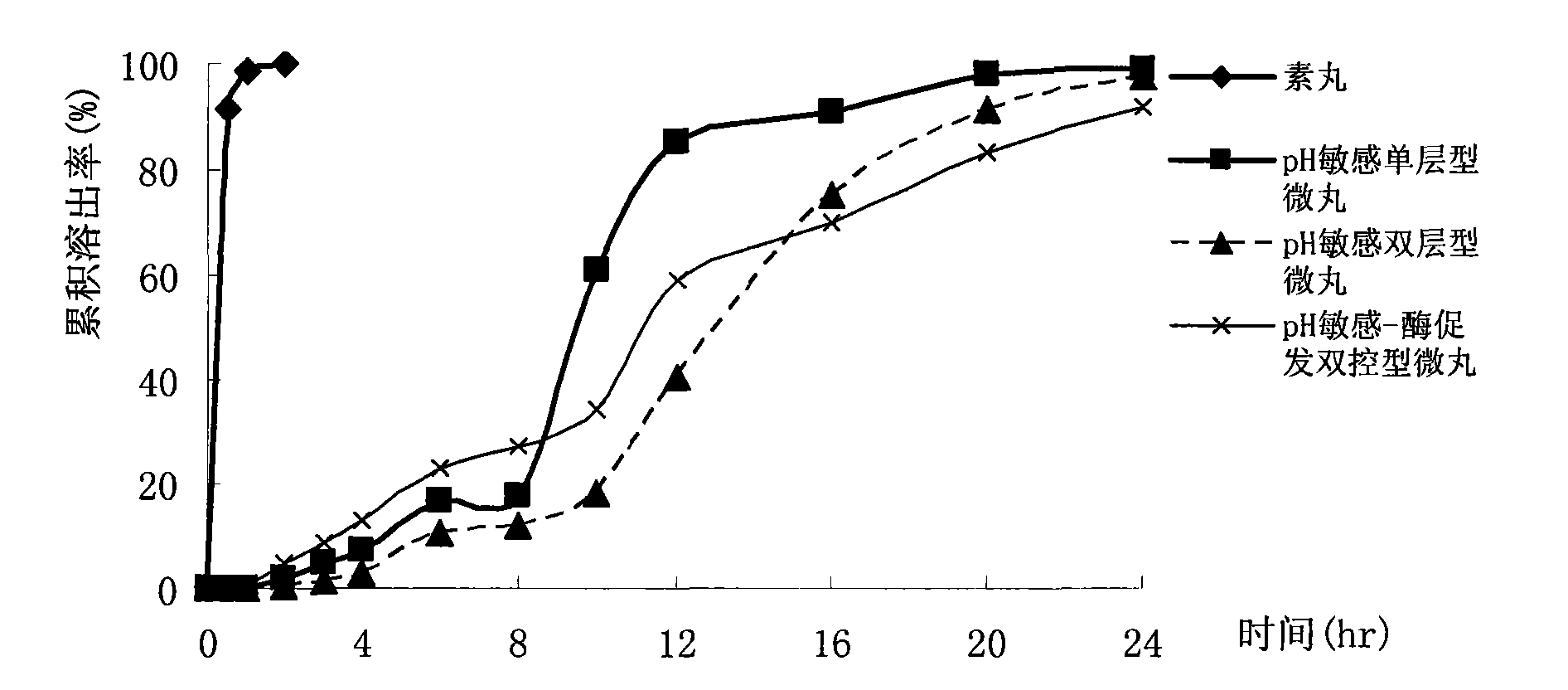
[0059] 2.1 pH-sensitive single-layer coated pellets
[0060] Matrine extract 51mg (matrine 50mg) oxymatrine extract 153mg (oxymatrine 150mg)
[0061] Microcrystalline Cellulose 755mg Sodium Carboxymethyl Starch 61mg
[0062] Coating 306mg
[0063]
[0064] Total 1326mg
[0065] 2.2 pH-sensitive double-layer coated pellets
[0066] Matrine extract 51mg Oxymatrine extract 153mg
[0067] Microcr...
Embodiment 3
[0078] Example 3 Screening test of pharmaceutical excipients and preparation process of the present invention
[0079] 1. Preparation of the medicine gastric-colon separate release capsule of the present invention
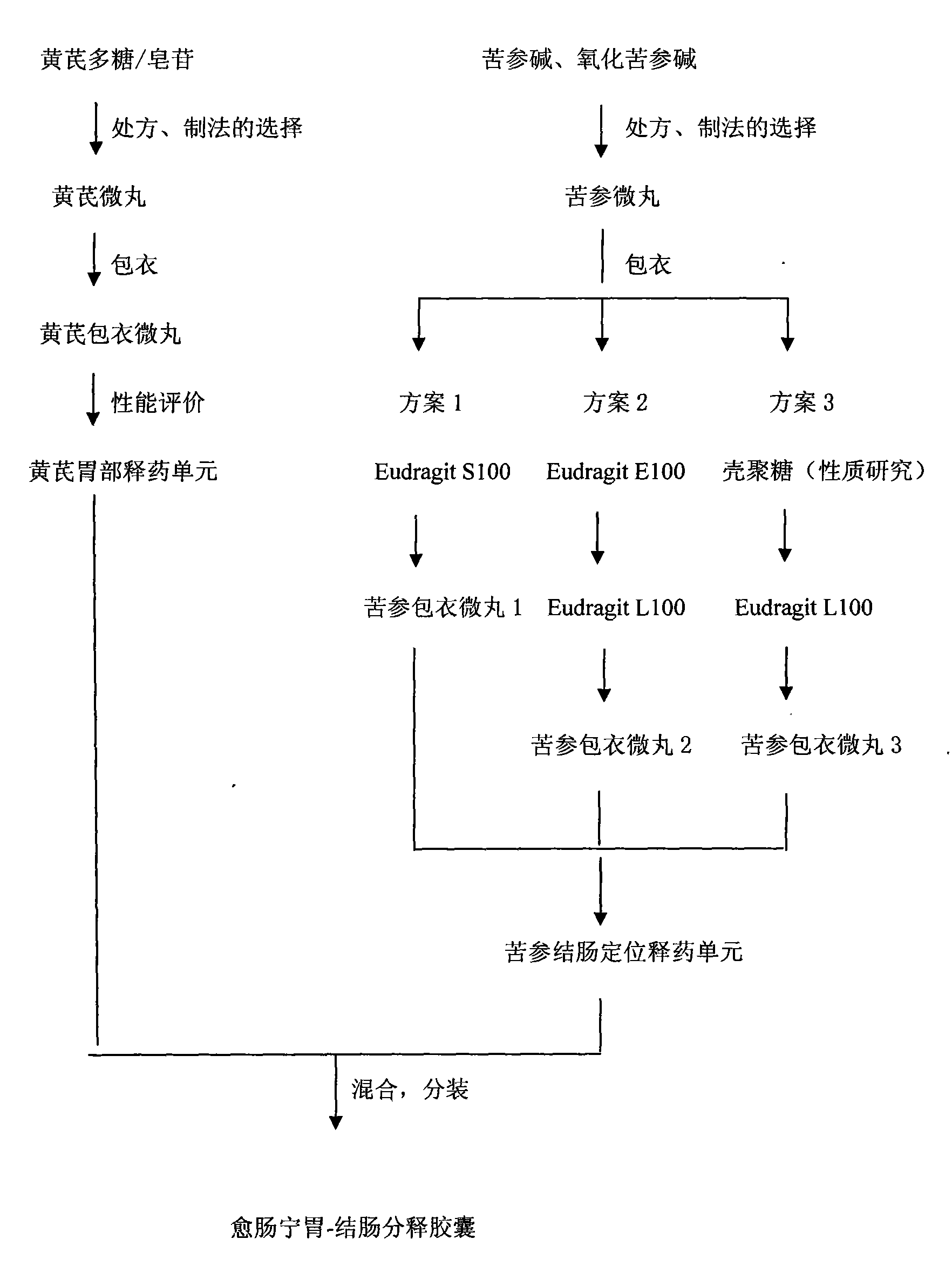
[0080] The drug of the present invention is divided into two parts, in which astragaloside and polysaccharide are prepared into a preparation unit that can release the drug quickly in the stomach, and matrine and oxymatrine are prepared into a preparation unit with colon-specific drug release performance. The two preparation units are mixed and assembled into a compound drug delivery system containing two drug release behaviors.
[0081] See the specific process route figure 1 .
[0082] 1 Instruments and reagents:
[0083] 1.1 Instrument
[0084] Spherical pellet granulator (Institute of Chemical Machinery, East China University of Science and Technology)
[0085] Fragility monitor (FT-2000 type, Tianjin Sixin Technology Co., Ltd.)
[0086] Powder flow tester (GTB type, EWEKA...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com