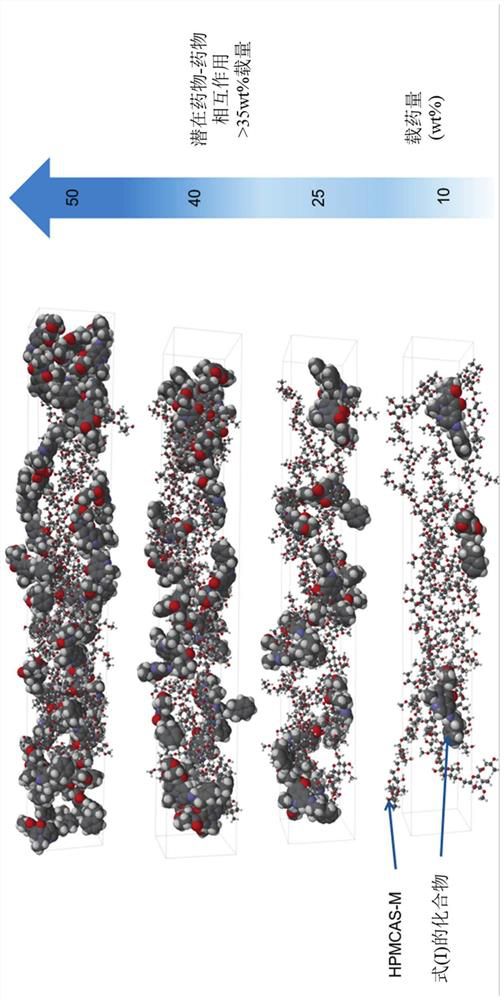
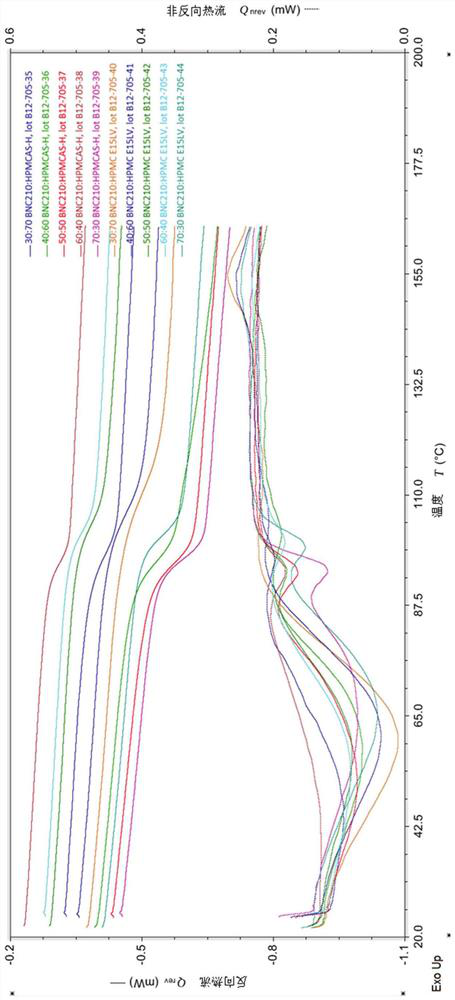
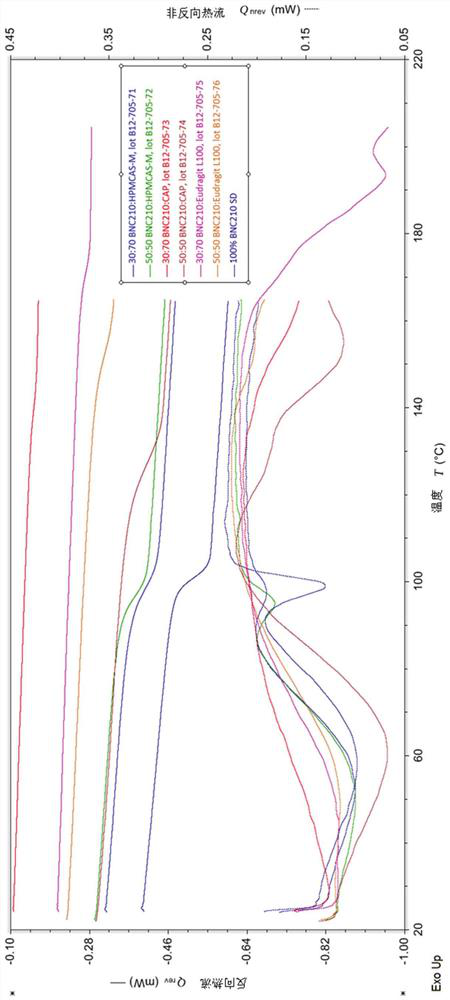
Therapeutic formulations and uses thereof
A technology of crystallization inhibitors and tablets, which can be used in pill delivery, medical preparations containing non-active ingredients, medical preparations containing active ingredients, etc., and can solve problems affecting API activity and bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0228] Example 1 (Ex1): Preparation of a representative spray-dried dispersion (SDD) composition of the present invention
[0229] Approximately 150g of the compound (I) (also referred to as "BNC210") is dissolved in a mixture of 4kg of dichloromethane and methanol (80:20% w / w). An appropriate amount of polymer (Table 1) was added to the solution, the mixture was stirred to give a homogeneous solution. Alternatively, additional excipients such as SLS sodium lauryl sulfate are added. Spray dry the solution using a Büchi B290 spray dryer with two fluid nozzles. The representative composition and physical appearance of the spray-dried dispersion powder are given in Table 1.
[0230]
[0231]
Embodiment 2
[0232] Example 2 (Ex2): Preparation of a representative hot melt extrusion (HME) dispersion composition of the present invention
[0233]Using Turbula Blender, about 6g of compound (I) (also known as "BNC210") and HPMCAS-H polymers were mixed, the blend was de-blocked through 25 meshes and blended again. The blended mixture is fed down at a screw speed of 200 rpm into a HAAKE Mini CTW extruder with a reverse screw rotation configuration of 165 °C or 175 °C or 180 °C and pressed by a mold. Optionally, use a dry cold but roller to quench the extrudate to cool. Grind the extrudate through a 60 mesh sieve.
[0234] The representative composition of the hot melt extruded extrusion and its physical appearance are given in Table 2.
[0235]
[0236]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com