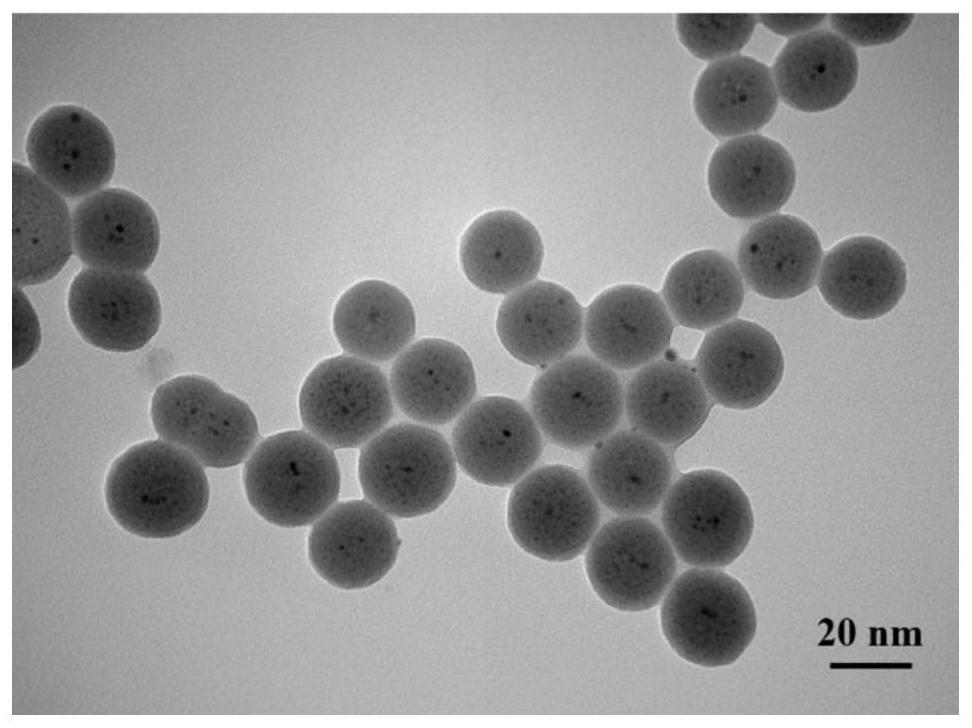
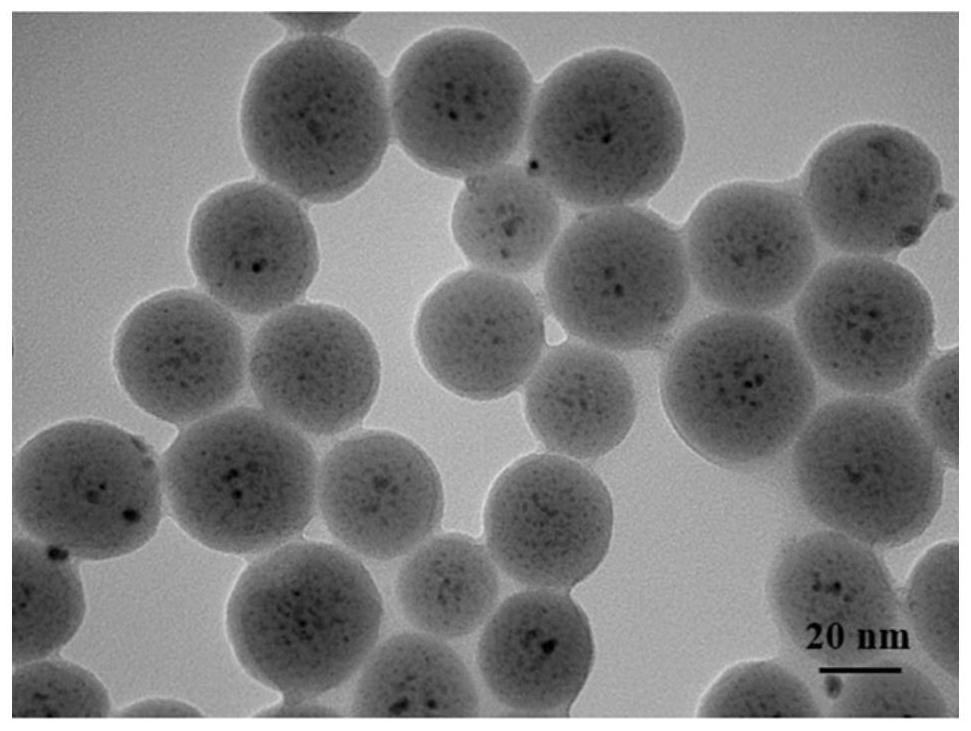
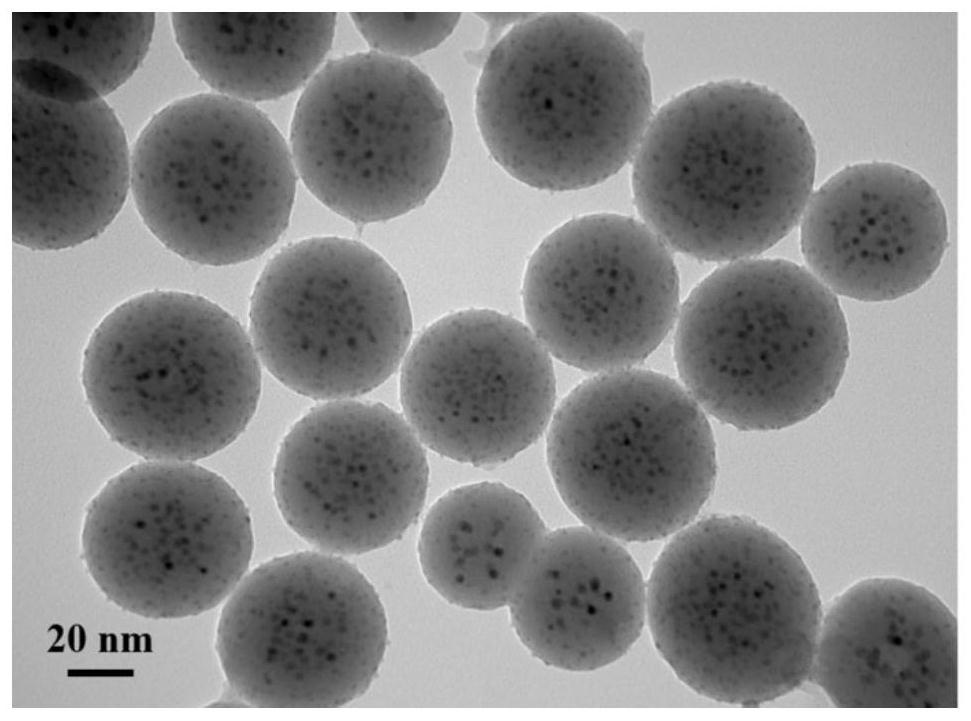
Nickel-oxide composite catalyst for high-selectivity preparation of iodo-arylamine from iodo-aromatic nitro compound and application of nickel-oxide composite catalyst
An arylnitro compound and composite catalyst technology, which is used in the preparation of amino compounds, metal/metal oxide/metal hydroxide catalysts, and organic compounds, etc. In order to achieve the effects of mild conditions, high stability and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation method: add Triton X-100, n-butanol and cyclohexane to analytically pure ammonia water, stir at room temperature until it becomes clear, then add nickel nitrate aqueous solution with a concentration of 0.1mol / L, and a concentration of 0.1mol / L. The aqueous solution of cerium nitrate was continuously stirred for 30 minutes, then tetraethyl orthosilicate was added dropwise, and the reaction was continued for 2 hours; finally, anhydrous ethanol was added to react for 10 minutes to obtain a reaction solution. Then, the obtained reaction solution was transferred to a sonicator for 10 min and then transferred to a centrifuge tube, centrifuged at 8000 rpm for 20 min, and the liquid phase after centrifugation was removed by pouring. under drying for 15h. After the obtained dried sample was cooled to room temperature, it was ground into powder. The calcination was carried out in a tube furnace at a calcination temperature of 500 °C for 6 h to obtain NiO-CeO 2 / SiO ...
Embodiment 2
[0030] The Ni-CeO prepared in Example 1 2 / SiO 2 As a hydrogenation reduction catalyst, it is used in the reaction of m-nitroiodobenzene hydrogenation to m-iodoaniline.
[0031] Add the prepared Ni-CeO to the reactor 2 / SiO 2 97mg (taking 1mg of active component as the calculation standard), take 1mmol of m-nitroiodobenzene and 10mL of anhydrous methanol and place it in an autoclave. After replacing the air, fill the reactor with hydrogen to 2MPa, and the ratio of the raw material to the hydrogen in the control reaction system is 20 (P(H) 2 ) / C(raw material)). The reaction was carried out at 140°C for 2 hours. The conversion rate of m-nitroiodobenzene was 93%, the selectivity of m-iodoaniline was 93%, and the yield was 86%.
Embodiment 3
[0033] Preparation method: the 58. Add n-butanol and cyclohexane to the analytically pure ammonia water, stir at room temperature until it becomes clear, then add a nickel nitrate aqueous solution with a concentration of 0.3 mol / L and an aqueous ammonium metavanadate solution with a concentration of 0.3 mol / L, and continue After stirring for 40 min, tetraethyl orthosilicate was added dropwise, and the reaction was continued for 2.5 h; finally, anhydrous ethanol was added to react for 15 min to obtain a reaction solution. Afterwards, the obtained reaction solution was transferred to a sonicator for 10 min and then transferred to a centrifuge tube, centrifuged at 10,000 rpm for 10 min, poured to remove the centrifuged liquid phase, and the obtained solid phase was washed with absolute ethanol at 90° C. under drying for 12h. After the obtained dried sample was cooled to room temperature, it was ground into powder. The calcination was carried out in a tube furnace, and the calc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap