Pharmaceutical composition containing FLT3 inhibitor and chemotherapeutic agent for treating acute myelogenous leukemia
An acute myeloid system and composition technology, applied in the direction of drug combinations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as poor prognosis and limited long-term curative effect
Pending Publication Date: 2022-06-21
HANMI PHARMA
View PDF3 Cites 0 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
In addition, with conventional acute myeloid leukemia (AML) standard chemotherapy, targeting AML stem / progenitor cells is impossible, so the disease often recurs in patients, and thus there is a problem of limited long-term efficacy (Non-Patent Document 2)
Treatment of FLT3- AML patients with ITD mutations also exhibit poorer prognosis (Non-Patent Document 4)
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example 1
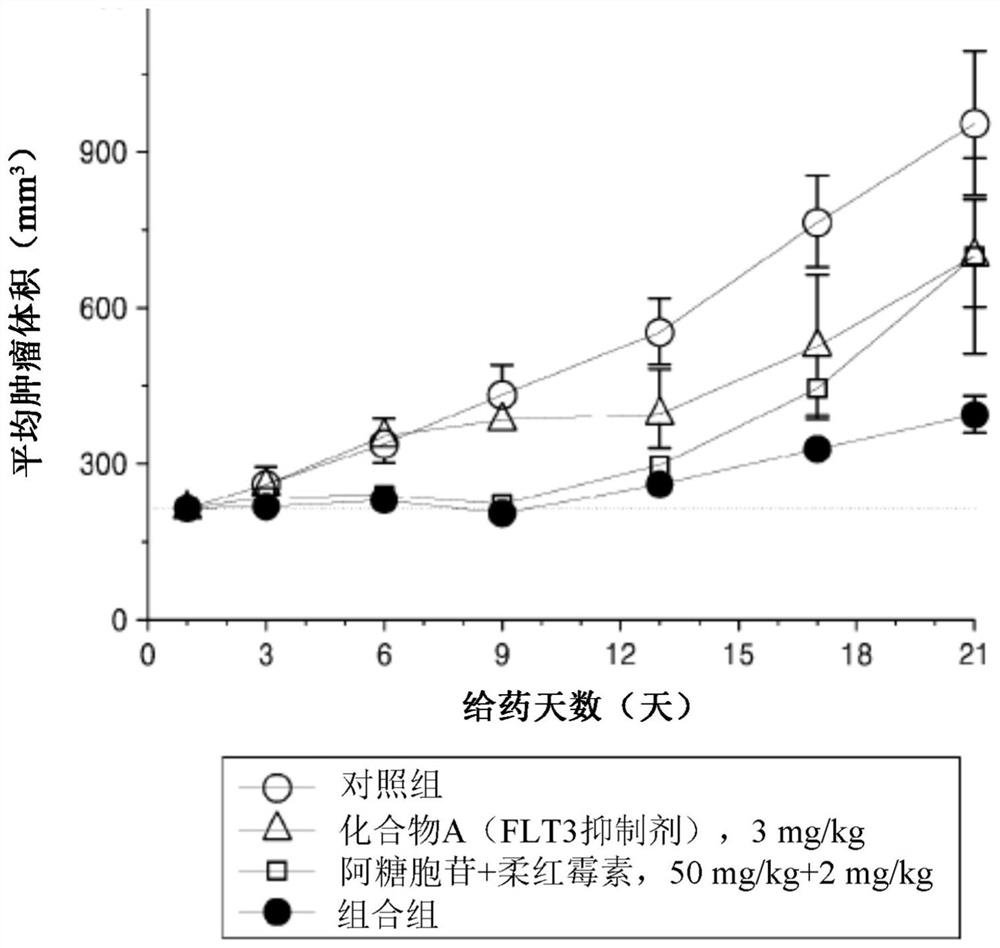
[0151] MV-4-11 cell line xenograft mouse model
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
Provided are pharmaceutical compositions for the treatment of acute myelogenous leukemia (AML); and a method for treating acute myelogenous leukemia by using the pharmaceutical composition. The pharmaceutical composition comprises a therapeutically effective combination of: an Fms-like tyrosine kinase-3 (FLT3) inhibitor, or a pharmaceutically acceptable salt or solvate thereof; and a chemotherapeutic agent or a pharmaceutically acceptable salt or solvate thereof.
Description
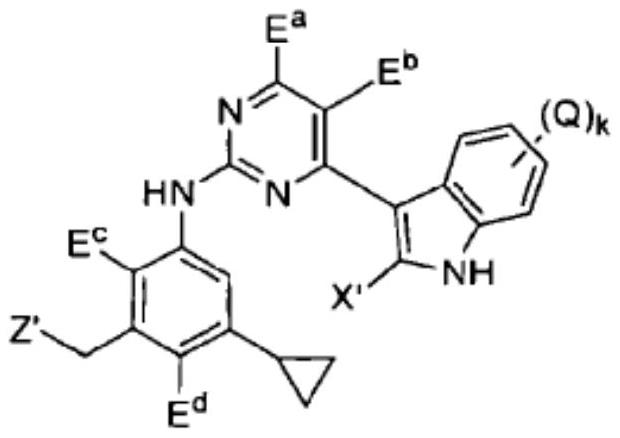
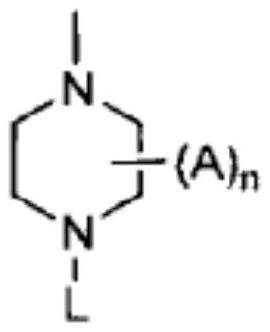
technical field [0001] The present invention relates to pharmaceutical compositions for the treatment of acute myeloid leukemia comprising effective therapeutic compositions of Fms-like tyrosine kinase-3 (FLT3) inhibitors and chemotherapeutic agents; and methods of treatment using such compositions. Background technique [0002] Fms-like tyrosine kinase-3 (FLT3) is one of the most commonly mutated genes in acute myeloid leukemia (AML). Mutant FLT3 (mutant FLT3) refers to a mutation expressed in leukemia cells present in a subset of acute myeloid leukemia (AML) patients. Activating mutations in FLT3, such as intragenic tandem duplication (ITD) in the proximal domain, appear in about 25-30% of newly diagnosed AML cases (Patent Document 1). FLT3 mutation is known to occur in about one-third of acute myeloid leukemia (AML) patients (Non-Patent Document 1). [0003] Although several FLT3 inhibitors are clinically available, drug-resistant leukocytes have been observed in AML pa...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K31/506A61K31/496A61K45/06A61P35/02
CPCA61K31/506A61P35/02A61K45/06A61K31/00A61K31/496A61K2300/00
Inventor 裵仁焕宋芝英崔载律安永吉
Owner HANMI PHARMA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com