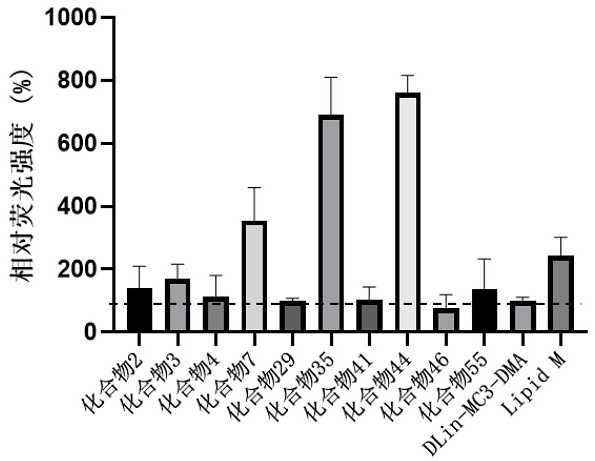
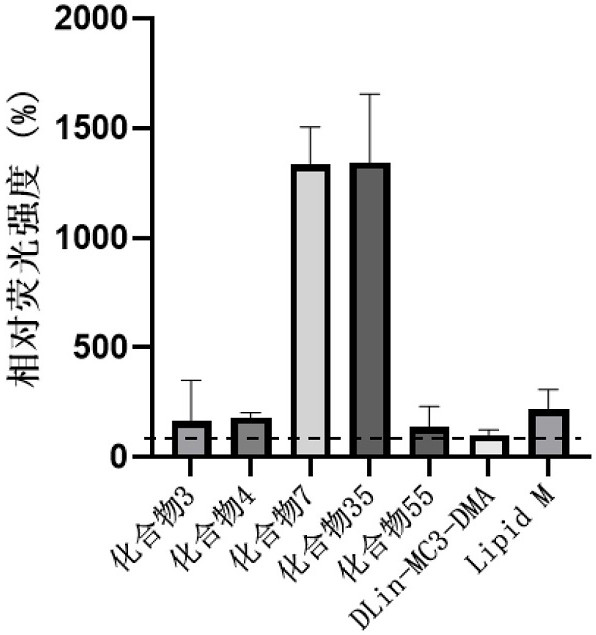
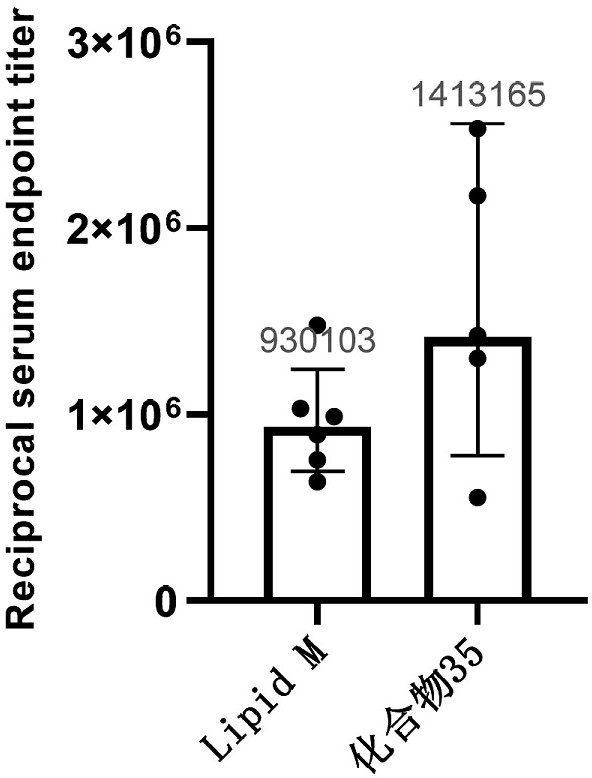
Cationic lipid compounds and compositions and uses for delivery of nucleic acids
A technology for cationic lipids and nucleic acid delivery, applied in drug combination, liposome delivery, organic chemistry, etc., can solve the problems of difficulty in entering target cells, short circulation time of naked mRNA, easy to be degraded, etc., to improve the clearance rate in vivo , The effect of less residue in the body and low carrier toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] Synthesis of Compound 2
[0078]
[0079] step 1:
[0080]To a solution of compound 2-1 (3.00 g) in tert-butanol (20 mL) were added 1-decanol (3.23 g) and cesium carbonate (11.1 g) in this order. After the solution was stirred at room temperature for 4 hours, a spot plate (petroleum ether:ethyl acetate=10:1) showed the formation of new spots. The reaction solution was filtered, and the concentrated crude product of the obtained filtrate was purified by column chromatography (silica gel column, eluent was a petroleum ether solution containing 0-10% ethyl acetate (volume percentage)) to obtain compound 2-2 (3.93 g , 69% yield).
[0081] Step 2:
[0082] To a solution of compound 2-2 (3.00 g) in THF (20 mL) and water was added lithium hydroxide (860 mg), and the mixture was stirred at 60° C. for 16 hours. TLC showed the formation of dots with increasing polarity. The reaction mixture was concentrated to remove tetrahydrofuran, diluted with water, extracted once wit...
Embodiment 2
[0093] Synthesis of Compound 3
[0094]
[0095]
[0096] step 1:
[0097] To a solution of compound 3-1 (3.00 g) in tert-butanol (20 mL) were added 1-decanol (4.45 g) and cesium carbonate (15.3 g) in this order. After the mixture was stirred at room temperature for 4 hours, a spot plate (petroleum ether:ethyl acetate=10:1) showed the formation of new spots. The reaction solution was filtered to obtain the crude product after the filtrate was concentrated, and the crude product was purified by column chromatography (silica gel column, eluent was a petroleum ether solution containing 0-10% ethyl acetate (volume percentage)) to obtain compound 3- 2 (3.1 g, 46% yield).
[0098] Step 2:
[0099] To a solution of compound 3-2 (3.00 g) in THF (20 mL) and water was added lithium hydroxide (860 mg), and the mixture was stirred at 60° C. for 16 hours. TLC (petroleum ether:ethyl acetate=10:1) showed the generation of a point with increased polarity. The reaction mixture was c...
Embodiment 3
[0106] Synthesis of compound 4
[0107]
[0108]
[0109] step 1:
[0110] To a solution of compound 4-1 (3.00 g) in tert-butanol (20 mL) were added 1-decanol (4.6 g) and cesium carbonate (16.1 g) in this order. After the mixture was stirred at room temperature for 4 hours, a spot plate (petroleum ether:ethyl acetate=10:1) showed the formation of new spots. The reaction solution was filtered to obtain a crude product after the filtrate was concentrated. The crude product was purified by column chromatography (silica gel column, eluent was a petroleum ether solution containing 0-10% ethyl acetate (volume percentage)) to obtain compound 4- 2 (3.0 g, 47.3% yield).
[0111] Step 2:
[0112]To a solution of compound 4-2 (3.00 g) in THF (20 mL) and water was added lithium hydroxide (860 mg), and the mixture was stirred at 60° C. for 16 hours. TLC (petroleum ether:ethyl acetate=10:1) showed the generation of a point with increased polarity. The reaction mixture was concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com