Preparation method of salt-spray-resistant modified polyaspartic acid ester, salt-spray-resistant modified polyaspartic acid ester and coating
An aspartic acid ester and salt spray-resistant technology, which is applied in polyurea/polyurethane coatings, anti-corrosion coatings, coatings, etc., can solve the problems of shortening the construction time of products, small steric hindrance of amino groups, and poor storage stability, etc. problem, to achieve the effect of long construction operation time, low viscosity and long curing time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
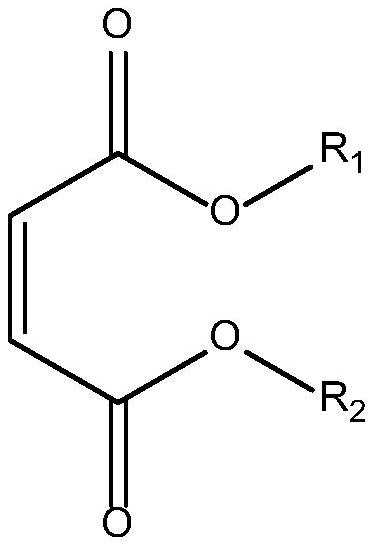
[0054] Take a 1L four-neck glass flask, add 210g (1mol) of 4,4'-diaminodicyclohexylmethane, and under the protection of nitrogen, add diethyl maleate 258 (1.5mol) dropwise, and the dropwise addition is completed in 3 hours, wherein , the double bond equivalent in diethyl maleate: the amine group equivalent in 4,4'-diaminodicyclohexylmethane=0.75:1;
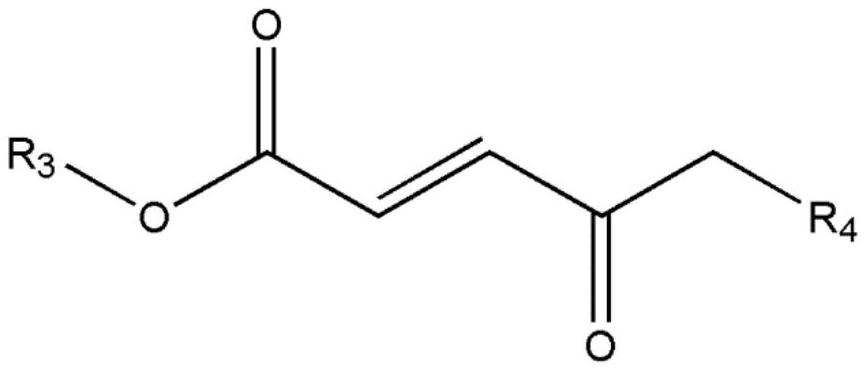
[0055] After the dropwise addition, react at 60°C for 6 hours, then add 185g (0.5mol) of NC-513 and heat up to 100°C, and react at 100°C for 6 hours to obtain salt spray-resistant modified polyaspartate;
[0056] The obtained salt spray-resistant modified polyaspartate had a secondary amine group equivalent of 326.5 and a viscosity of 4500 mPa·s (25° C.).
Embodiment 2
[0058] Take a 1L four-neck glass flask, add 238 g (1 mol) of 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane, and dropwise add 244.8 g (1.7 g of dimethyl maleate) under the protection of nitrogen. mol), the dropwise addition was completed in 4 hours, wherein, the double bond equivalent in dimethyl maleate: the amine group equivalent in 3,3'-dimethyl-4,4'-diaminodicyclohexylmethane=0.85:1 ;
[0059] After the dropwise addition, the reaction was carried out at 80 °C for 24 hours, then 111 g (0.3 mol) of NC-513 was added, the temperature was raised to 90 °C, and the reaction was carried out at 90 °C for 5 hours to obtain a salt spray-resistant modified polyaspartic acid ester;
[0060] The obtained salt spray-resistant modified polyaspartate had a secondary amine group equivalent of 296.9 and a viscosity of 2500 mPa·s (25° C.).
Embodiment 3
[0062] Take a 1L four-neck glass flask, add 170 g (1 mol) of isophorone diamine, and under the protection of nitrogen, add 244.8 g (1.7 mol) of dimethyl maleate dropwise, and the dropwise addition is completed in 5 hours. Double bond equivalent in dimethyl ester: amine group equivalent in isophorone diamine=0.85:1;
[0063] After the dropwise addition, the reaction was carried out at 85 °C for 48 hours, then 63 g (0.15 mol) of NC-514 was added and the temperature was raised to 90 °C, and the reaction was carried out at 90 °C for 6 hours to obtain a salt spray-resistant modified polyaspartic acid ester;
[0064] The obtained salt spray-resistant modified polyaspartate had a secondary amine group equivalent of 312.3 and a viscosity of 4200 mPa·s (25° C.).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap