Tacrine derivative as CDKs inhibitor and application thereof
A derivative, the technology of tacrine, applied in the field of organic compound synthesis and pharmaceutical applications, can solve the problems of neglecting anti-cancer activity and low anti-proliferation activity of tumor cells, and achieve the effect of novel structure and high anti-proliferation activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
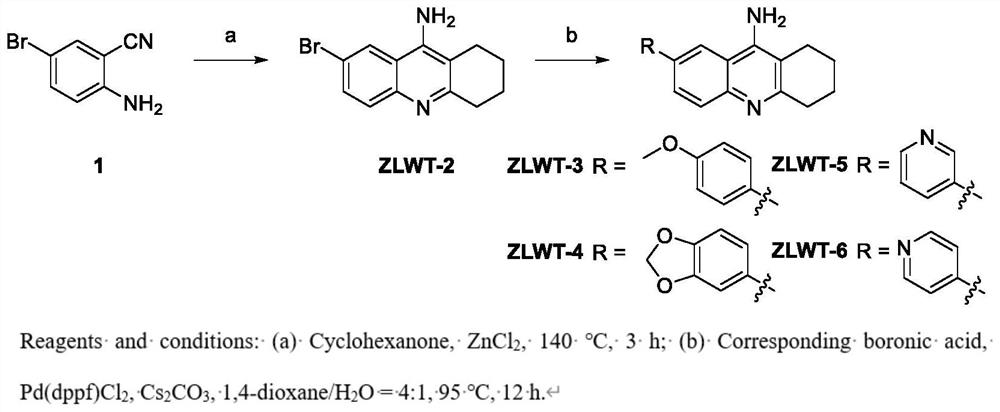
[0033] Example 1 7-Bromo-1,2,3,4-tetrahydroacridine-9-amine (ZLWT-2)
[0034] like figure 1 As shown, to a solution of 2-amino-5-bromobenzonitrile (Compound 1) (1 equiv) in cyclohexanone (50 mL) was added anhydrous ZnCl 2 (3 equivalents). The mixture was stirred at 140°C for 3 hours. After cooling, after filtration, the filtrate was concentrated and purified by silica gel chromatography to obtain the target compound ZLWT-2. Yield 76%, off-white solid. 1 H NMR (400MHz, DMSO-d 6 )δ8.48(d,J=1.9Hz,1H),7.66-7.55(m,2H),6.71(s,2H),2.82(t,J=5.8Hz,2H),2.57-2.48(m,2H) ),1.81(dd,J=7.6,4.4Hz,4H).ESI-MS m / z 277.2[M+H] + .
Embodiment 2
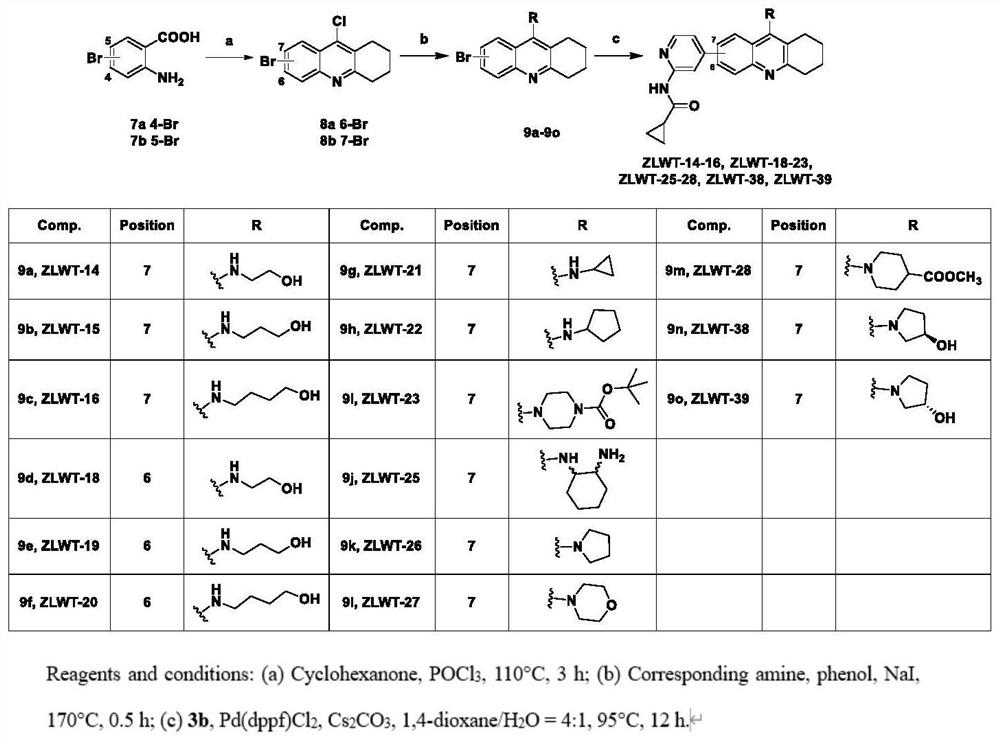
[0035] Example 2 6-Bromo-1,2,3,4-tetrahydroacridin-9-amine (Compound 11)
[0036] like Figure 4 As shown, 2-amino-4-bromobenzonitrile (compound 10) was used as the raw material, and the synthesis method was as in Example 1 to obtain compound 11. Yield 66%, off-white solid compound 11. 1 H NMR (400MHz, DMSO-d 6 )δ8.31(d, J=2.1Hz, 1H), 7.53-7.26(m, 2H), 6.58(s, 2H), 2.76(m, 2H), 2.64-2.52(m, 2H), 1.78(m ,4H).ESI-MS m / z277.1[M+H] + .
Embodiment 3
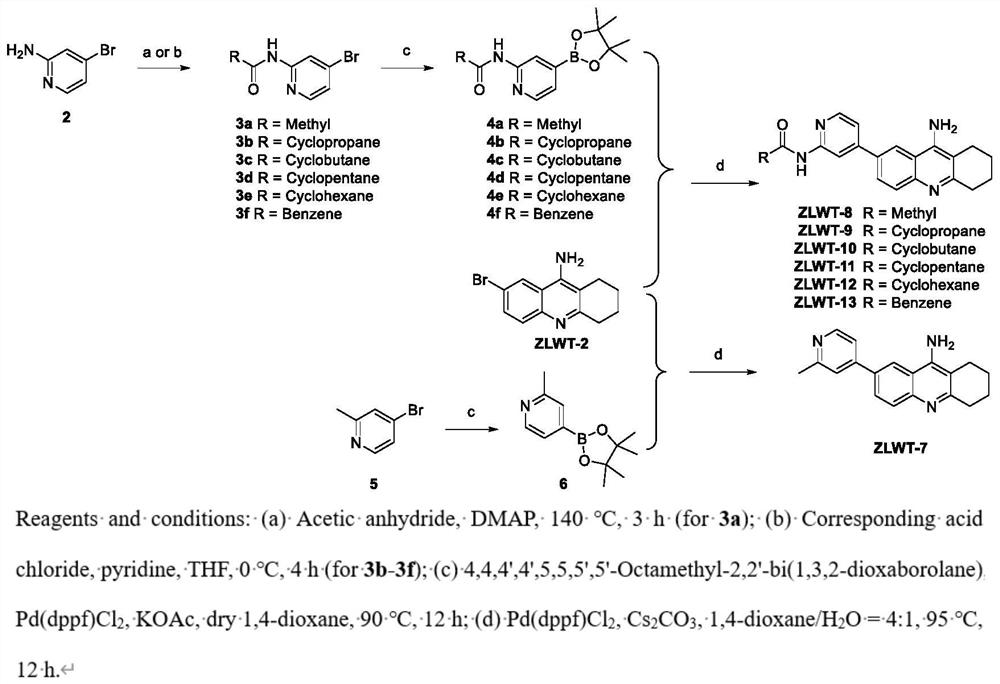
[0037] Example 3N-(4-Bromopyridin-2-yl)acetamide (Compound 3a)
[0038]
[0039] like figure 2 As indicated, to a solution of 4-bromo-2-aminopyridine (compound 2) (5 g, 28.90 mmol) in acetic anhydride (50 mL) was added DMAP (0.035 g, 0.3 mmol). After the mixture was stirred at 140°C for 6 hours, it was poured into ice water, neutralized with 2M NaOH, and filtered to obtain compound 3a as a white solid with a yield of 78%. ESI-MS m / z 214.8[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com