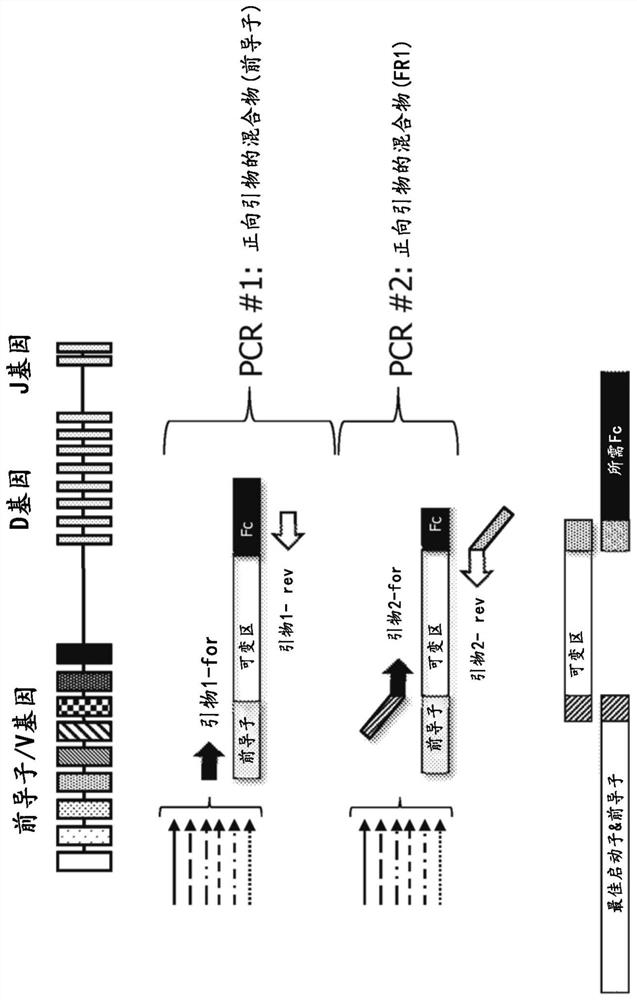
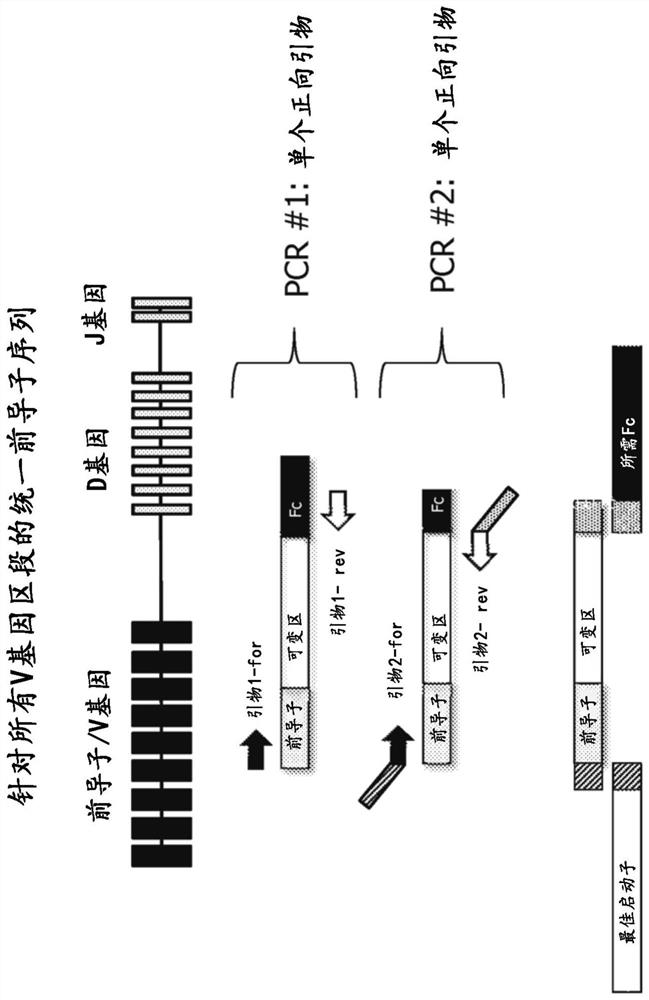
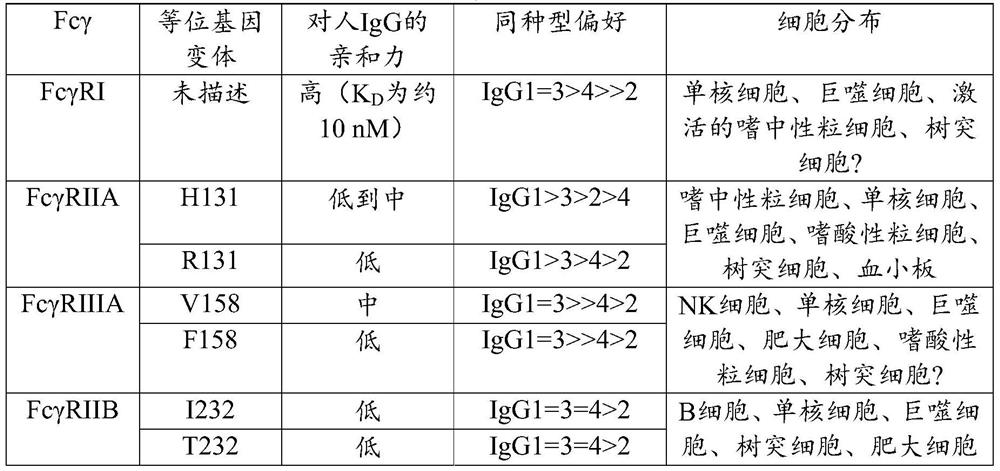
BCR transgenic mice with common leader sequence
A technology of transgenic animals and sequences, which can be used in medical preparations containing active ingredients, anti-animal/human immunoglobulins, and antibody medical components, etc., which can solve problems such as reducing the diversity of polyclonal antibody libraries.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Selection of the optimal leader peptide coding sequence
[0128] The optimal leader peptide sequences and optimal leader peptide coding sequences for use in the methods, constructs and animals of the present invention are selected as follows. Briefly, an initial set of potential leader peptide sequences was selected based on conservation and consensus of sequences within all leader sequence sets, frequency and usage in human antibody repertoires, and sequence diversity across the initial set was made maximize. Both amino acid and DNA sequences are also ranked based on their ubiquity in human antibody repertoires and in approved antibody drugs, based on the fact that such selection indicates that the sequences are biologically ideal and suitable for commercial antibody producing cells Systems and methods and therapeutic agents for use in humans. The recombinant protein expression efficiency of the set of leader peptides was then evaluated by pairwise evaluation of heav...
Embodiment 2
[0133] Selection of heavy and light chain V gene segments
[0134] V gene segments for use in the methods and mice of the present invention are selected as follows. Briefly, V gene segments for both heavy and light chains are based on their prevalence in human antibody repertoires, in antibody therapeutics that have entered Phase I clinical trials, and in approved antibody drugs. sex to choose. They were also assessed for chemical barriers (such as methionine (especially in CDRs) or presence of unpaired cysteines), as well as sequence barriers like DG and NG, presence of glycosylation sites and immunogenicity Sex - through experimental observations and computational predictions (e.g. by Immunogenicity Assessment Software, EpiVax Inc., Providence, R.I., USA).
[0135] After considering and balancing the above factors, for the heavy chain variable domain locus, the following 19 V gene segments (hIGHV) were selected: 3-23; 5-51; 3-7; 1-2; 1-69- 1;3-48;1-18;1-46;3-21;3-30;3-7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com