Recombination plasmid and application in disease prevention and control
A technology of recombining plasmids and diseases, applied in the field of biomedicine, to achieve the effect of preventing excessive scar formation, simple and convenient application, and promoting wound healing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Experimental Study of Using Recombinant Plasmid pUDKH Gene to Treat Limb Ischemia in Rats
[0059] 1. Construction and preparation of recombinant plasmids
[0060] (1) Construction of plasmid pUDKH
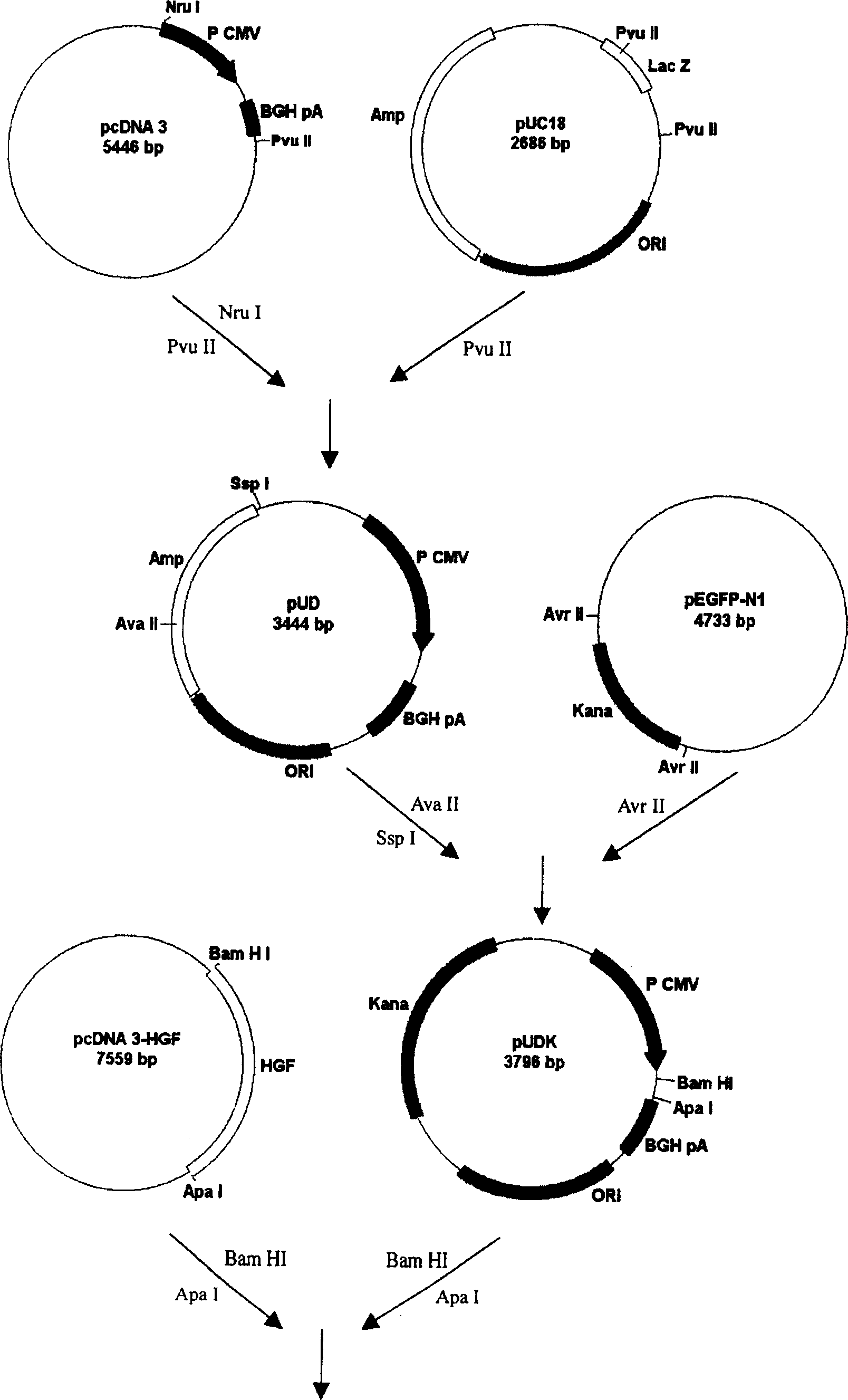
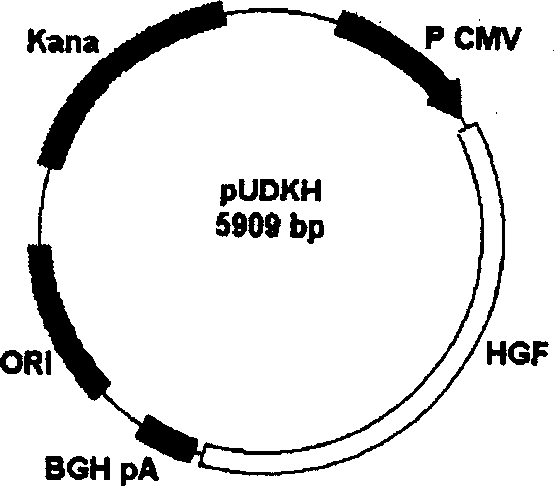
[0061] Build process like figure 1 : The pUC18 cloning vector was digested with PvuII, and a 2.3kb DNA fragment was recovered. pcDNA3 was digested with NruI and PvuII, and a 1 kb DNA fragment was recovered, which contained cytomegalovirus promoter, multiple cloning site and growth hormone terminator. The two fragments were connected to obtain plasmid pUD (3.3kb). Digest pUD with AvaII and SspI to recover a 2.7kb DNA fragment, and digest pEGFP-N1 with AvrII to recover a 1.1kb DNA fragment, which contains the complete kanamycin gene; connect the two recovered DNA fragments to obtain Plasmid pUDK (3.8kb). Then insert the cDNA of the human hepatocyte growth factor coding region into the multiple cloning site of the pUDK vector to obtain the recombinant vector pUD...
Embodiment 2
[0083] Example 2 Prediction of scar formation by recombinant plasmid pUDKH carrying human hepatocyte growth factor gene
[0084] Anti-effect
[0085] 1. Construction and large-scale purification of recombinant plasmid pUDKH
[0086] Same as Example 1
[0087] 2. Transfection of recombinant plasmid DNA into NIH3T3 cells
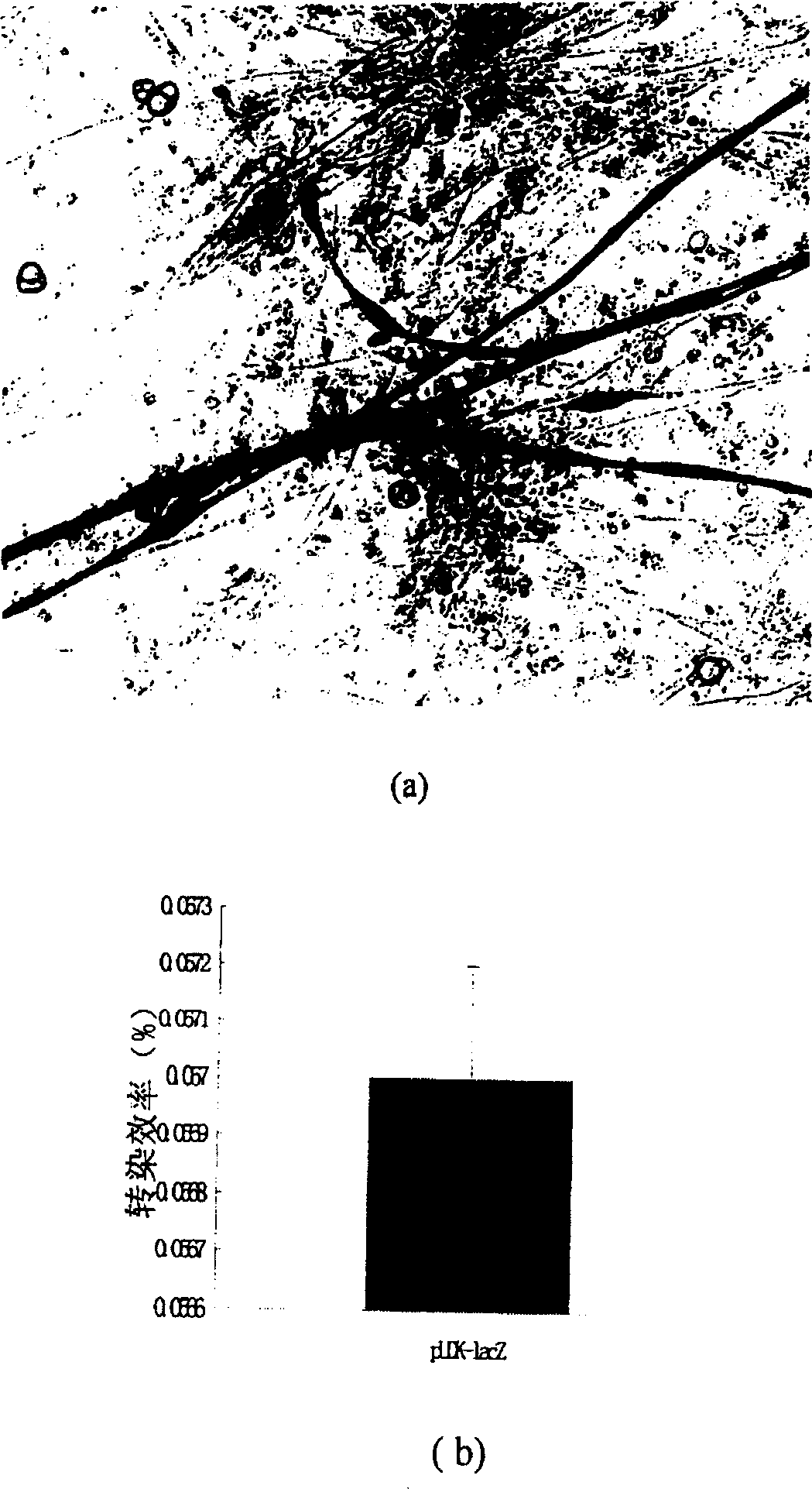
[0088] The plasmid DNA was transfected into NIH3T3 cells using the lipofectin method, and 5 micrograms of DNA + 20 microliters of liposomes were used for each transfection. After transfecting pUDK-lacZ, its transfection efficiency was 7.96%; after transfecting pUDKH (number of cells was 1.0×10 6 ), samples were taken at 24 hours, 48 hours and 72 hours after transfection, and the expression level of HGF was detected by ELISA method. The primary antibody was mouse anti-human HGF monoclonal antibody (R&D). The results showed that the expression of HGF could be detected in the supernatant of NIH3T3 cells transfected with pUDKH (such as Figure 4 , Figure...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com