Non-crystal hydrogenation catalyst for anthraquinone process of preparing hydrogen peroxide solution and its preparing method
A technology of hydrogen peroxide and catalyst, which is applied in the field of new catalyst for anthraquinone hydrogenation and its preparation, and can solve problems such as thickening of working fluid viscosity, high price of palladium catalyst, and high production cost of hydrogen peroxide
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Embodiment 1: the preparation of amorphous Ni-M-B alloy catalyst
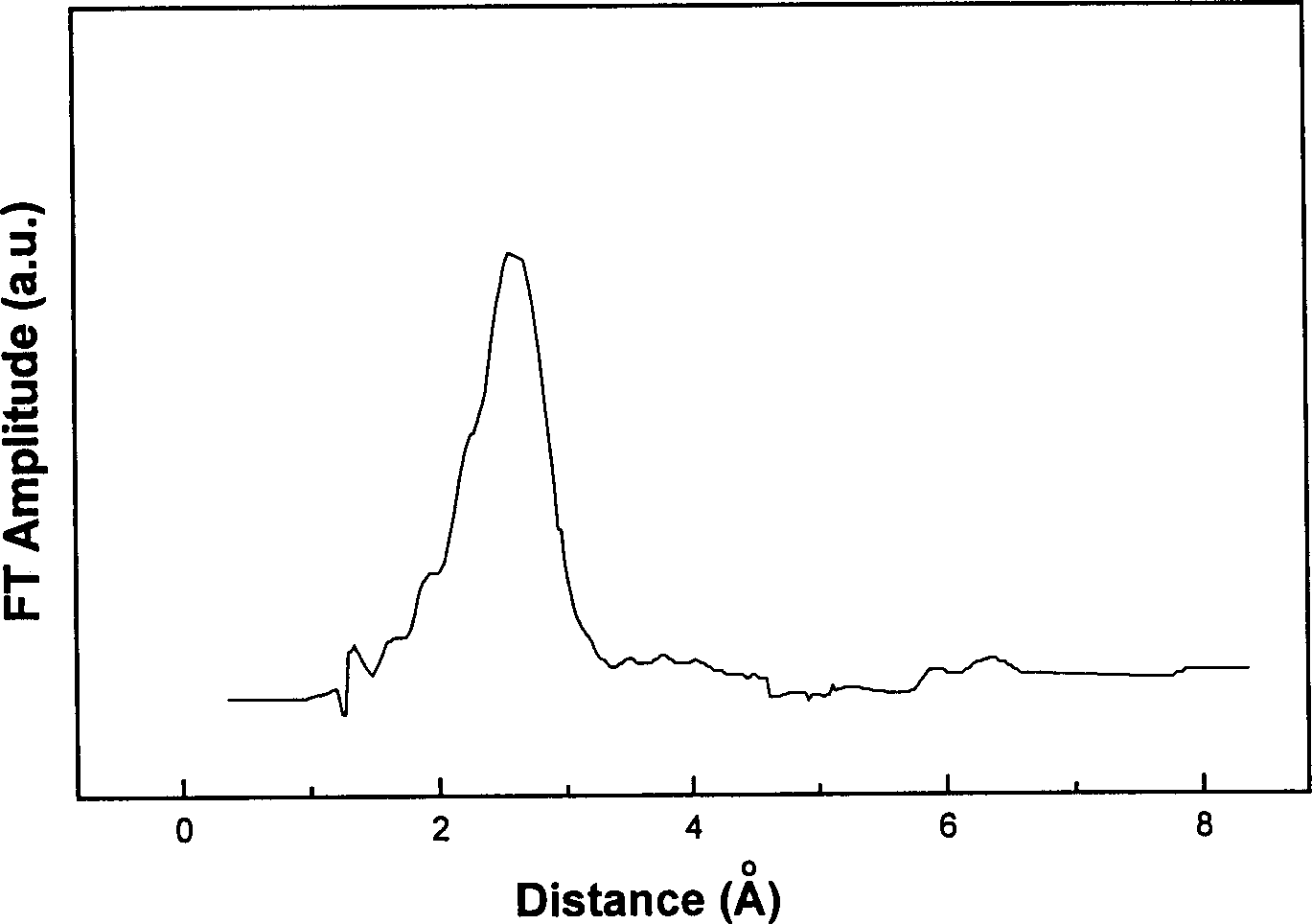
[0019] Na 2 MO 4 (5gM L -1 ) added to NiCl 2 Aqueous solution (0.42mol·L -1 ), after mixing evenly, place in an ice-water bath, slowly add KBH dropwise while stirring 4 Aqueous solution (2.0mol·L -1 ), if M is Cr, Mo, W, Ni-Cr-B, Ni-Mo-B, Ni-W-B catalysts are prepared respectively. The obtained black precipitate was separated by centrifugation, washed with deionized water until neutral, and then the water in the catalyst was replaced with absolute ethanol. Some characterization results of the catalysts are shown in Table 1.
Embodiment 2
[0020] Embodiment 2: the preparation of amorphous Ni-Cr-B alloy catalyst
[0021] Na 2 CrO 4 (5gM L -1 ) into NiCl 2 Aqueous solution (0.42mol·L -1 ), after mixing evenly, place in an ice-water bath, slowly add KBH dropwise while stirring 4 Aqueous solution (2.0mol·L-1 ), changing the added volume of sodium chromate can obtain catalysts with different chromium contents, which are respectively recorded as Ni-Cr-B-1, Ni-Cr-B-2, Ni-Cr-B-3, Ni-Cr-B- 4. The obtained black precipitate was separated by centrifugation, washed with deionized water until neutral, and then the water in the catalyst was replaced with absolute ethanol. Partial characterization results of the catalysts are shown in Table 2.
Embodiment 3
[0022] Embodiment 3: Anthraquinone hydrogenation activity test
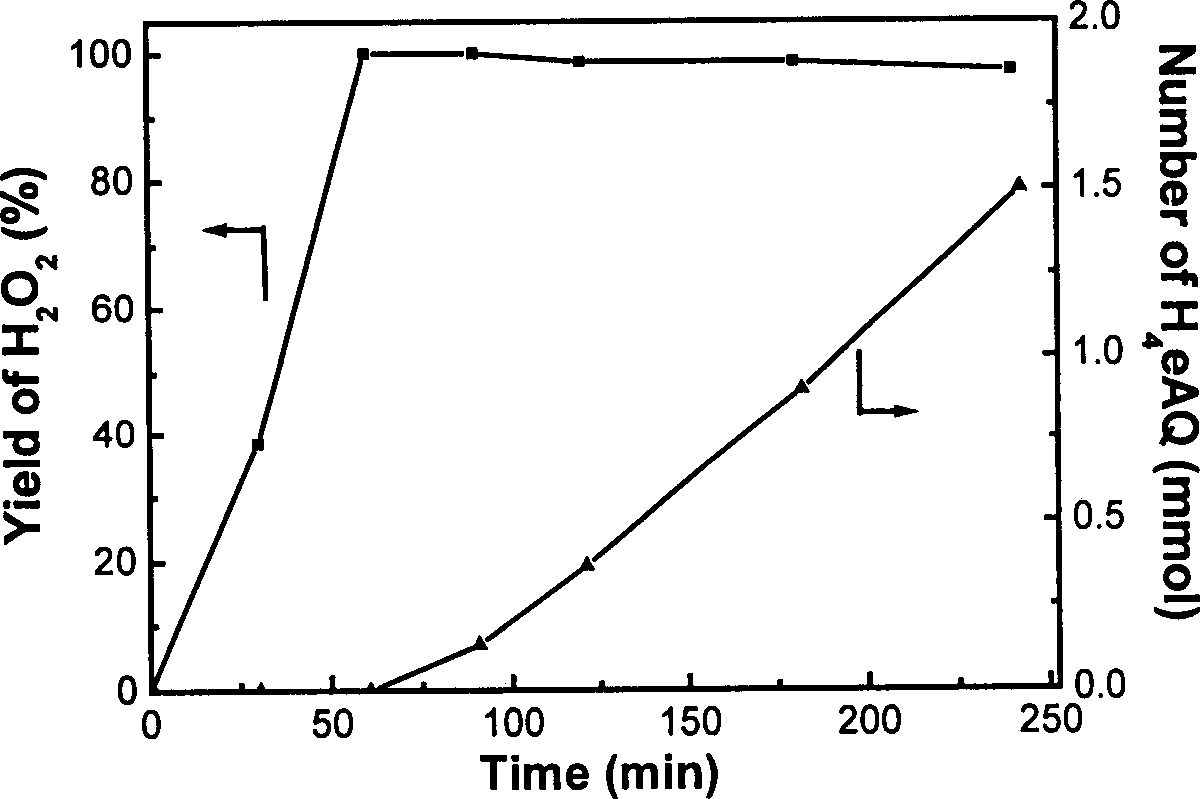
[0023] Dissolve 3.5g of anthraquinone in 70mL of a solvent made of heavy aromatics and trioctyl phosphate in a volume ratio of 7:3, and put it into the reactor together with 0.5g of Ni-Cr-B-2 catalyst, and fill it with The hydrogen pressure was introduced to 3atm, the reaction temperature was controlled at 50±2°C, and the stirring rate was 1000 rpm. The results obtained are listed in figure 2 : The yield of hydrogen peroxide reaches 100% in 60 minutes, and when the yield of hydrogen peroxide reaches the maximum, the selectivity to carbonyl hydrogenation also reaches 100%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com