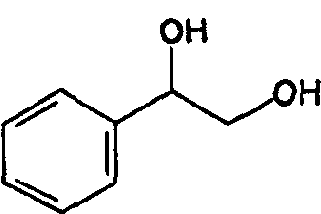
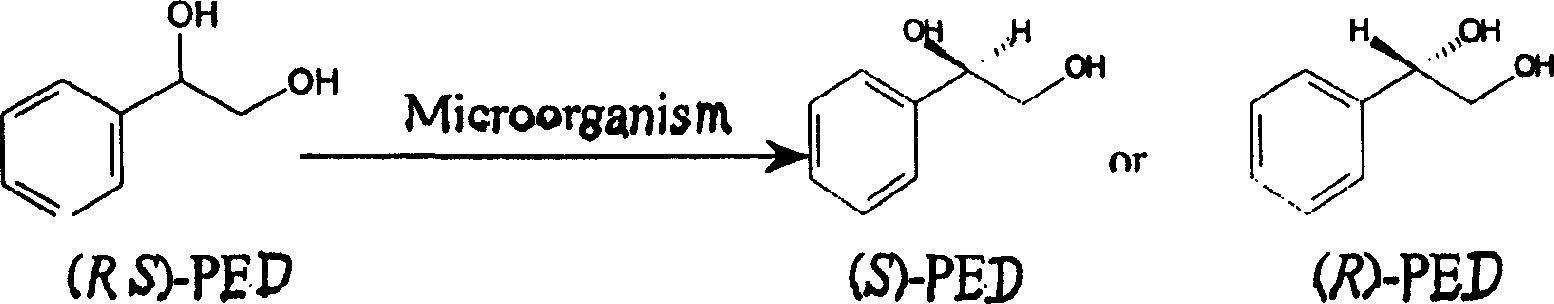
Method for preparing optical pure phenylethanediol by utilizing microbial stereoselectivity transformation and its special-purpose microbe
A technology of pure phenylethylene glycol and biological method, applied in the field of biological separation of racemic compounds, can solve the problems of low optical purity of products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0086] Whole-cell catalytic transformation of Candida parapsilosis C.parapsilosis CCTCC M203011:
[0087] In 2ml, 0.1mol / L potassium phosphate buffer solution (pH6.5), add 0.1g bacterial cells and 16mg racemic phenylethylene glycol, shake and react on a constant temperature shaker at 33°C for 48 hours, after the reaction , the mixture was centrifuged. The product (S)-PED had an optical purity of 98.80% e.e. and a yield of 92.86%.
Embodiment 2
[0089] Whole-cell catalytic transformation of Bacillus polymyxa CCTCC M203010:
[0090] In 2ml, 0.1mol / L potassium phosphate buffer solution (pH7.0), add 0.16g bacterial cells and 10mg racemic phenylethylene glycol, shake and react on a constant temperature shaker at 33°C for 60 hours, after the reaction , the mixture was centrifuged. The optical purity of the product (S)-PED increased to 95.61% e.e., and the yield increased to 47.98%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com