Organic flux system in hydrogen peroxide producing process
An organic solvent and production process technology, applied in the field of organic solvent systems in the production process of hydrogen peroxide, can solve problems such as low production capacity, and achieve the effects of improving production capacity, reducing side reactions, and improving hydrogen efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
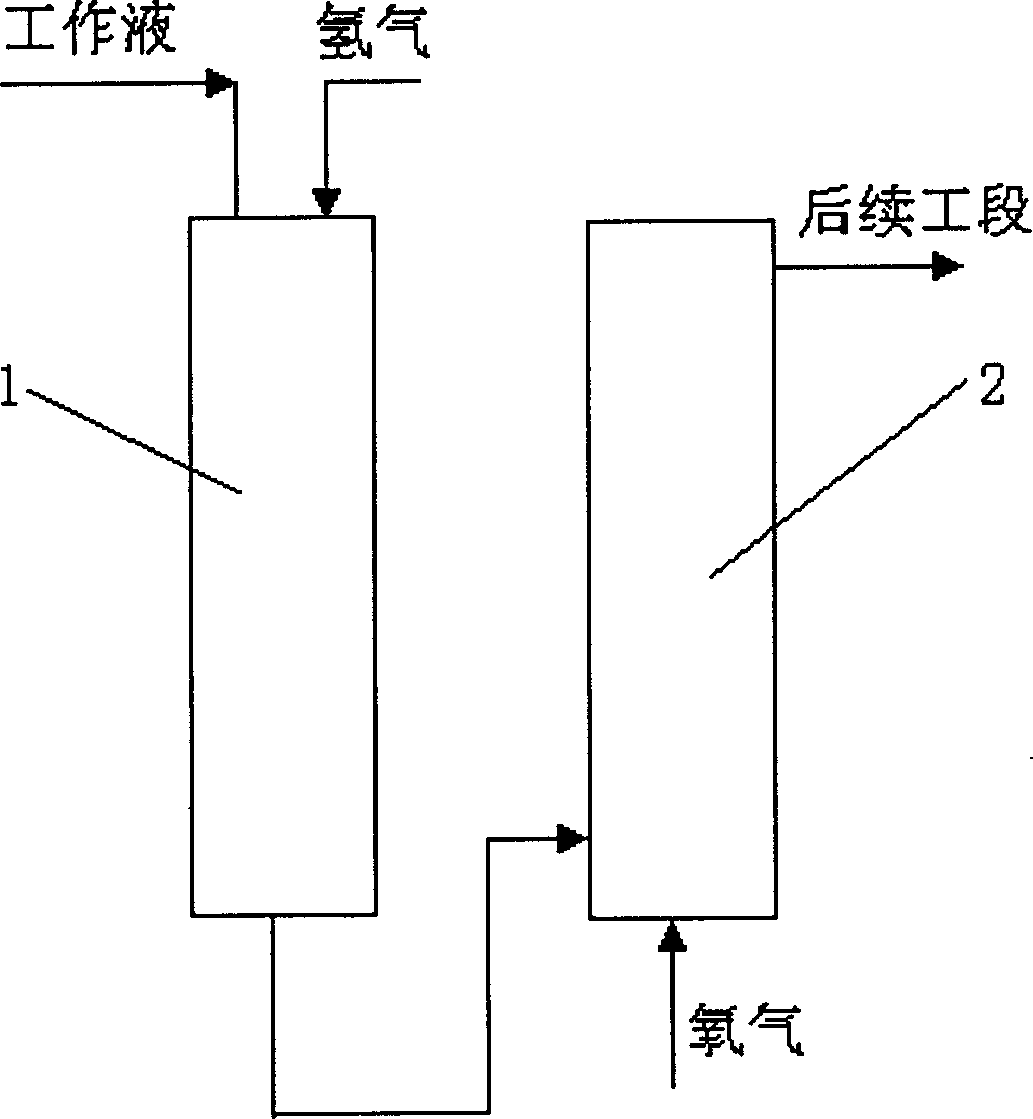
[0029] use figure 1 The procedure for testing and evaluating the solvent system of the present invention.
[0030] Using 60L C 9 ~C 10 A solvent system was prepared from a mixture of aromatic hydrocarbons, 12.5L trioctyl phosphate and 26.5L 2-methylcyclohexyl acetate. At room temperature, the solubility of 2-ethylanthraquinone is 172g / L, and the catalyst is Pd-M / Al 2 o 3 , wherein, M is Na, and the content is 0.3wt%.
[0031] see figure 1 , the working liquid and hydrogen are sent to the hydrogenation tower 1 to hydrogenate the 2-ethylanthraquinone in the working liquid, the hydrogenation liquid enters the oxidation tower 2, is oxidized by the air entering the oxidation tower 2, and generates hydrogen peroxide aqueous solution, and then enters the subsequent section. The hydrogenation tower 1 is a fixed trickle bed reactor with a diameter of 40mm and a height of 1425mm, and the catalyst packing height is 400mm.
[0032] The hydrogen efficiency is 9.93g / L, the hydrogenat...
Embodiment 2
[0034] Adopt the condition of embodiment 1, change catalyst into Pd-M / SiO 2 system, M is Na, and the content is 0.3wt%. The hydrogen efficiency is 10.03g / L, the hydrogenation reaction temperature is 65°C, and the oxidation time is 4 minutes.
Embodiment 3
[0036] use figure 1 The procedure for testing and evaluating the solvent system of the present invention.
[0037] Using 72.4L C 9 ~C 10 A solvent system was prepared from a mixture of aromatic hydrocarbons, 22.5L trioctyl phosphate and 5.1L 2-methylcyclohexyl acetate. At room temperature, the solubility of 2-ethylanthraquinone is 152g / L, and the catalyst is Pd-M / Al 2 o 3 , wherein, M is Na, and the content is 0.3wt%.
[0038] see figure 1 , the working liquid and hydrogen are sent to the hydrogenation tower 1 to hydrogenate the 2-ethylanthraquinone in the working liquid, the hydrogenation liquid enters the oxidation tower 2, is oxidized by the air entering the oxidation tower 2, and generates hydrogen peroxide aqueous solution, and then enters the subsequent section. The hydrogenation tower 1 is a fixed trickle bed reactor with a diameter of 40mm and a height of 1425mm, and the catalyst packing height is 400mm.
[0039] The hydrogen efficiency is 8.86g / L, the hydrogena...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com