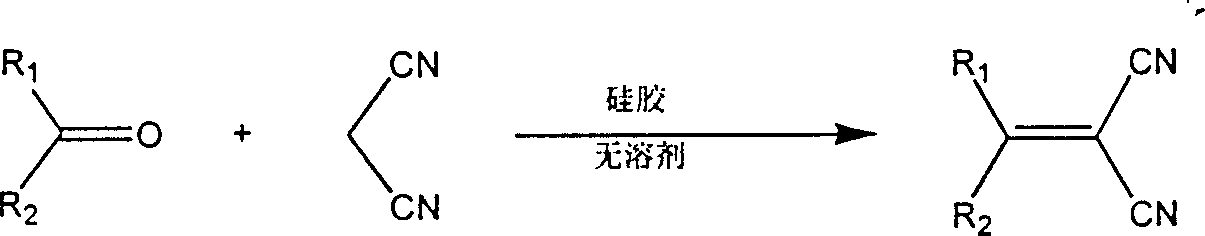
Microwave radiation ketone-nitrile condensation method adopting amine acetate catalysis
A technology of microwave radiation and ammonium acetate, applied in chemical instruments and methods, preparation of carboxylic acid nitrile, preparation of organic compounds, etc., can solve the problems of slow reaction and low yield, and achieve short reaction time, high efficiency and no solvent pollution effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] This embodiment takes the condensation of benzophenone and malononitrile as an example.
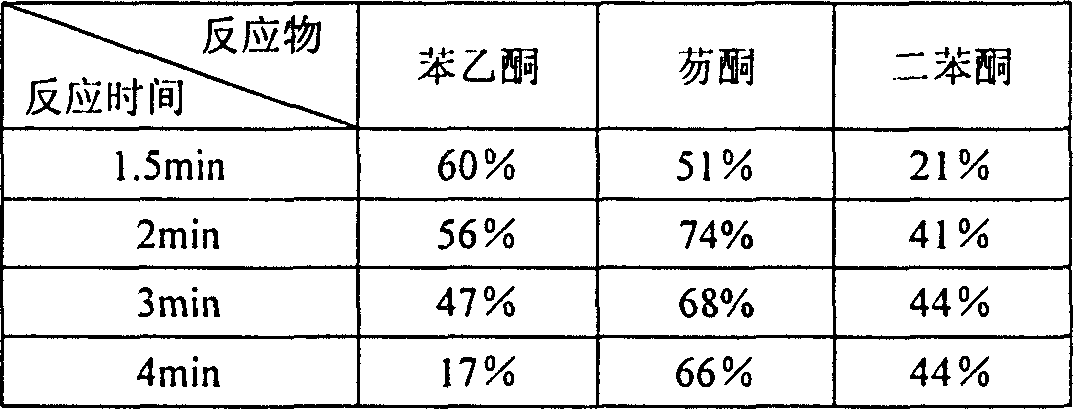
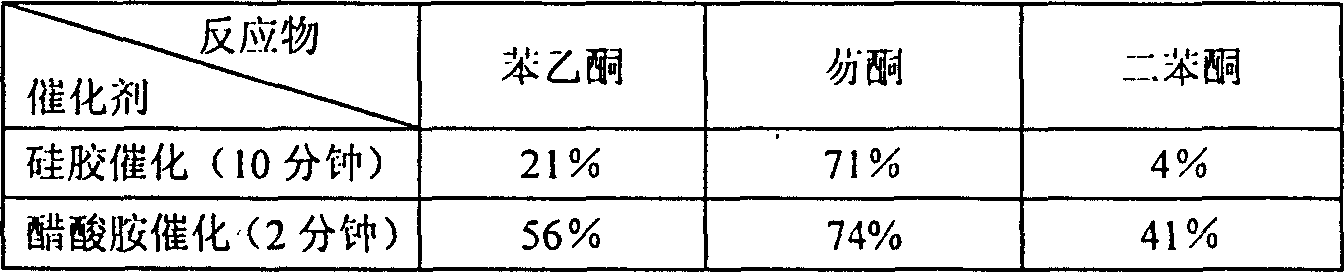
[0014] Put 100 mg of benzophenone, equimolar amounts of malononitrile, and equimolar amounts of ammonium acetate into open dry glass test tubes to mix the reaction mixture thoroughly. Microwave for 3 minutes. After the reaction was completed, the reaction mixture was cooled. The cooled reaction mixture was fully dissolved in dichloromethane, and the ammonium acetate was removed using a suction filter funnel, and the dichloromethane suction filtrate was retained; the filtered ammonium acetate was washed twice with dichloromethane to fully collect the product. The above two dichloromethane solutions were combined, transferred to a round bottom flask, and the dichloromethane was removed by a rotary evaporator to obtain a crude product.
[0015] Finally, the obtained crude product was further separated and purified by silica gel column chromatography to obtain a ketone-nitrile conden...
Embodiment 2
[0017] This embodiment takes the condensation of fluorenone and malononitrile as an example.
[0018] Put 100 mg of fluorenone, equimolar amounts of malononitrile, and equimolar amounts of ammonium acetate into open dry glass test tubes to mix the reaction mixture thoroughly. Then, put the test tubes into a microwave oven and use a 300-watt microwave Radiation for 2 minutes. After the reaction was completed, the reaction mixture was cooled. Fully dissolve the cooled reaction mixture in dichloromethane, remove ammonium acetate using a suction filter funnel, retain the dichloromethane suction filtrate; and rinse the filtered ammonium acetate twice with dichloromethane. The above two dichloromethane solutions were combined. Transfer to a round bottom flask and remove the dichloromethane with a rotary evaporator to give the crude product.
[0019] Finally, the obtained crude product was recrystallized with ethanol, and the calculated yield of the ketone-nitrile condensation pro...
Embodiment 3
[0021] This embodiment This embodiment takes the condensation of acetophenone and malononitrile as an example.
[0022] Put 100 mg of acetophenone, equimolar amounts of malononitrile, and equimolar amounts of ammonium acetate into open dry glass test tubes to mix the reaction mixture thoroughly. Microwave irradiation for 1.5 minutes. After the reaction was completed, the reaction mixture was cooled. Fully dissolve the cooled reaction mixture in dichloromethane, remove ammonium acetate using a suction filter funnel, retain the dichloromethane suction filtrate; and rinse the filtered ammonium acetate twice with dichloromethane. The above two dichloromethane solutions were combined, transferred to a round bottom flask, and the dichloromethane was removed by a rotary evaporator to obtain a crude product.
[0023] Finally, the obtained crude product was further separated by silica gel column chromatography, and the calculated yield of the ketone-nitrile condensation product was 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com