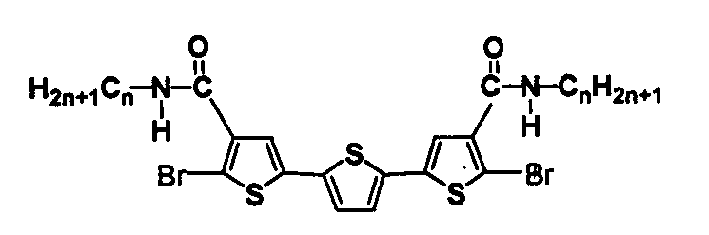
Liquid crystal compound contg. oligothiophene skeleton, preparing process thereof
A liquid crystal compound, oligothiophene technology, applied in chemical instruments and methods, liquid crystal materials, etc., can solve the problem of oligothiophene liquid crystal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] The first step is at -78℃, N 2 A solution (30 ml) of DBr3T (5,5″-dibromo-2,2′:5′,2″-trithiophene, 2.0 g, 4.9 mmol) in tetrahydrofuran (THF) was added to diisopropylamine under protection Lithium (LDA, 19.6mmol) in tetrahydrofuran (THF, 20ml) solution, stirred for 1 hour;
[0018] In the second step, excess dry ice (CO 2 ), at -78°C, N 2 Under protection, continue to react for 3 hours, then warm up to room temperature, continue to react for 3 hours;
[0019] The third step is to add dilute hydrochloric acid to the reaction solution, continue to react for 5 hours, remove THF and water by filtration, wash with water, and dry;
[0020] The fourth step the above product (0.5g, 1.0mmol) was added to thionyl chloride (SOCl 2 , 0.24g, 2.0mmol) in 1,2-dichloroethane solution (80ml), reflux reaction for 5 hours, remove solvent;
[0021] The fifth step was to mix the above product with octadecylamine (0.5g, 1.9mmol) at 0°C under N 2 Under protection, react in the dichloromet...
Embodiment 2
[0023] The first step is at -78℃, N 2 A solution (40 ml) of DBr3T (5,5″-dibromo-2,2′:5′,2″-trithiophene, 3.0 g, 7.4 mmol) in tetrahydrofuran (THF) was added to diisopropylamine under protection Lithium (LDA, 29.6mmol) in tetrahydrofuran (THF, 30ml) solution, stirring reaction for 2 hours;
[0024] In the second step, excess dry ice (CO 2 ), at -78°C, N 2 Under protection, continue to react for 4 hours, then warm up to room temperature, continue to react for 4 hours;
[0025] The third step is to add dilute hydrochloric acid to the reaction solution, continue to react for 6 hours, filter to remove THF and water, wash with water, and dry;
[0026] The fourth step the above product (1.0g, 2.0mmol) was added to thionyl chloride (SOCl 2 , 0.48g, 4.0mmol) in 1,2-dichloroethane solution (100ml), reflux reaction for 7 hours, remove solvent;
[0027] The fifth step, the above product and hexadecylamine (0.9g, 3.7mmol) were mixed at 0°C under N 2 Under protection, react in dichlor...
Embodiment 3
[0029] The first step is at -78℃, N 2 A solution of DBr3T (5,5″-bisbromo-2,2′:5′,2″-trithiophene, 2.5 g, 6.1 mmol) in tetrahydrofuran (THF) (35 ml) was added to diisopropylamine under protection Lithium (LDA, 24.4mmol) in tetrahydrofuran (THF, 25ml) solution, stirring reaction for 3 hours;
[0030] In the second step, excess dry ice (CO 2 ), at -78°C, N 2 Under protection, continue to react for 5 hours, then warm up to room temperature, continue to react for 5 hours;
[0031] The third step is to add dilute hydrochloric acid to the reaction solution, continue to react for 8 hours, remove THF and water by filtration, wash with water, and dry;
[0032] The fourth step the above product (0.4g, 0.8mmol) was added to thionyl chloride (SOCl 2 , 0.19g, 1.6mmol) in 1,2-dichloroethane solution (60ml), reflux reaction for 8 hours, remove solvent;
[0033] The fifth step was to mix the above product with octaalkylamine (0.2g, 1.6mmol) at 0°C under N 2 Under protection, react in the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com