Mechanism for the deployment of endovascular implants
A technique for laying tubes and internal devices, which is applied in the field of embolizing vascular aneurysms and similar vascular abnormalities, and achieves the effects of easy repositioning, easy adaptation, and convenient and reliable separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
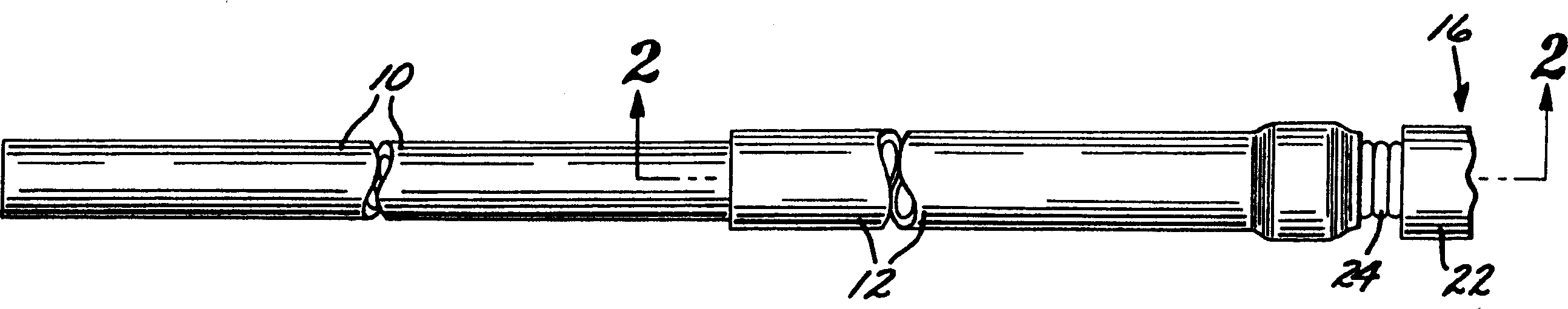
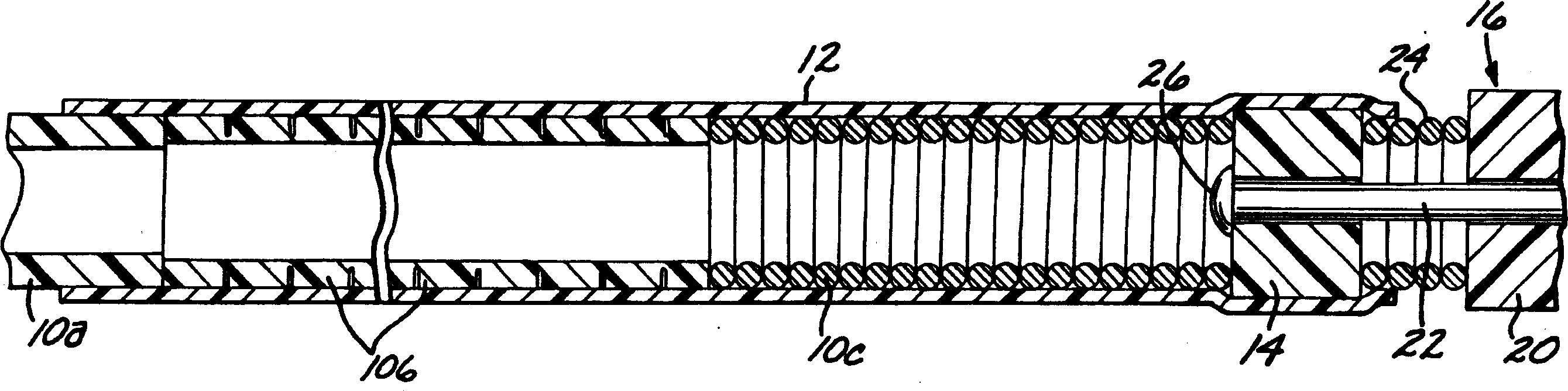
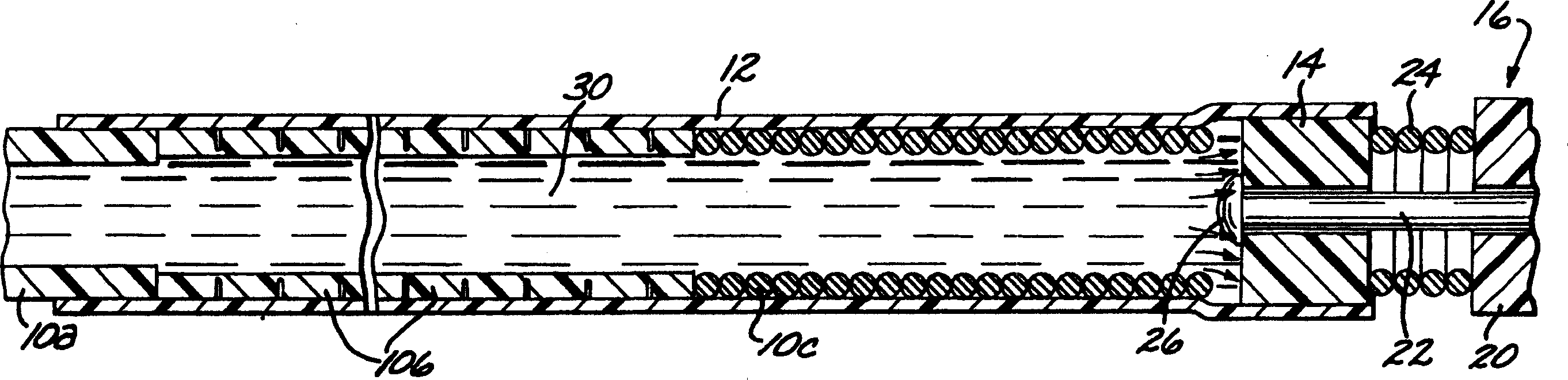
[0040] first reference figure 1 According to the present invention, a deployment device for an intravascular device comprises an elongated flexible hollow deployment tube 10 having an open proximal end 11 (see Figure 11 ) and end at the distal portion of the open distal end 13, with a continuous fluid passage cavity 15 formed between the proximal and distal ends. A retention sleeve 12 is fixed around the deployment pipe 10, said retention sleeve comprising a terminal extension 17 which extends a short distance past the distal end 13 of the deployment pipe. The deployment device also includes a connector 14 secured to the proximal end (only the proximal end of which is shown) of a filamentary intravascular device 16, which may be, for example, an embolic implant.
[0041] The deployment tube 10 is fabricated from stainless steel and is preferably formed in three sections, each sized to pass through a typical microcatheter. The proximal or main section 10a is the longest sect...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com