Methods of treating antibody-mediated pathologies using agents which inhibit CD 21
An antibody-mediated technology, applied in the direction of antibodies, pharmaceutical formulations, chemical instruments and methods, etc., can solve the problems of limited research on the role of CD21
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
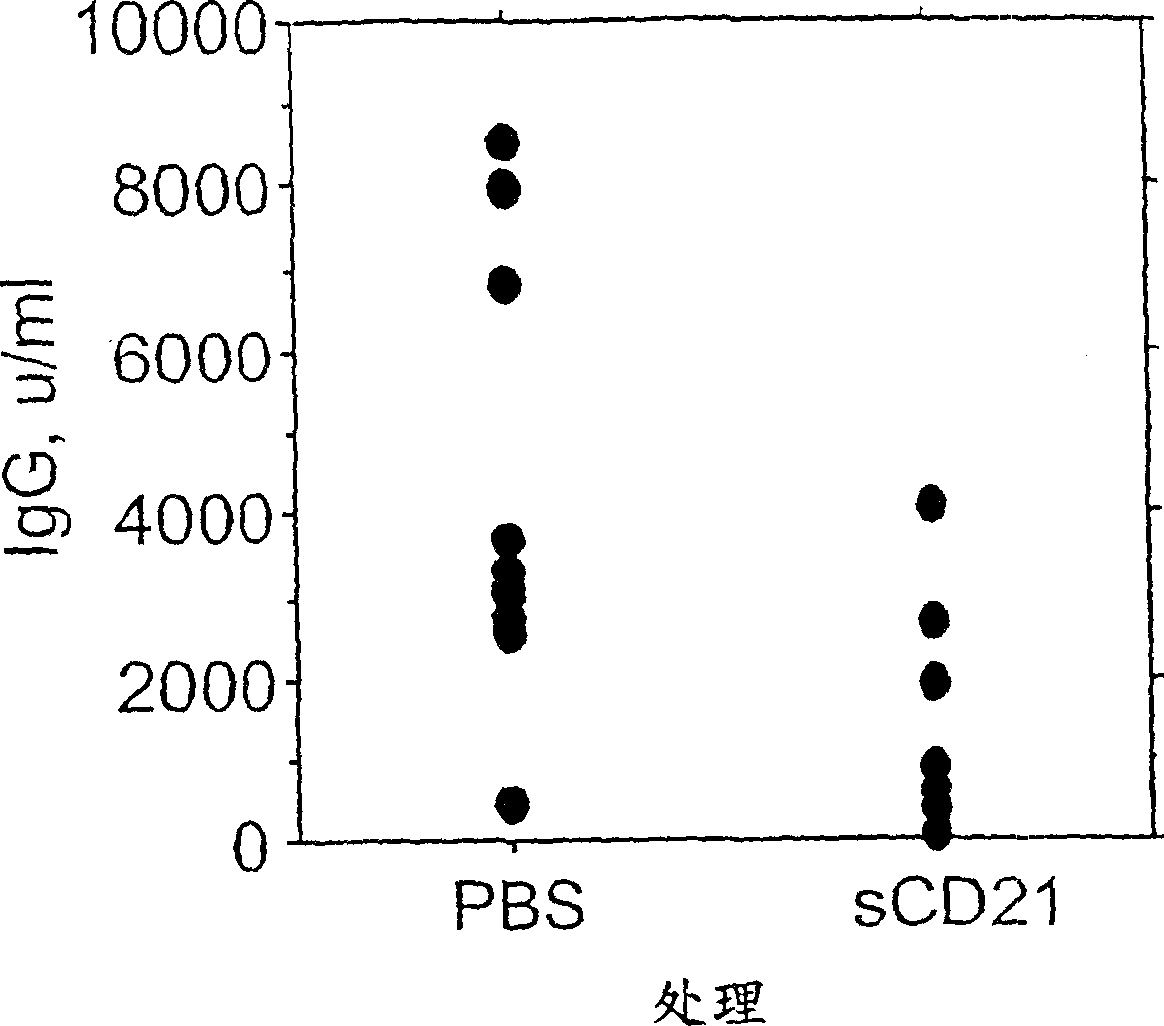
[0146] Example 1 Use of sCD21 to reduce the level of anti-dsDNA antibodies
[0147] C57 / B6 mice were sensitized with a model T cell-dependent antigen, which was coupled to (CA) at a ratio of 3.8 moles of oligonucleotide / mole of KLH 10 -(TG) 10 Composed of 20-mer double-stranded oligonucleotides on the keyhole: hemocyanin (KLH) (ON-KLH).
[0148] Mice were bled and subsequently administered intraperitoneally with 50 μg of alum-precipitated ON-KLH together with 2 × 10 9 A sacrificed Bordetella pertussis (B.Pertussis) organism was immunized. Fourteen days later, the mice were boosted with intraperitoneal administration of 10 μg ON-KLH. At the time of booster immunization, mice received 300 μg soluble CD21 (sCD21; Hebell et al. (1991) Science 254: 102) intravenously and 300 μg sCD21 intraperitoneally. Control mice received PBS. For the next 3 days, mice received 300 μg of sCD21 or PBS intravenously per day.
[0149] Mice were bled 7 days after the boost and IgG levels specif...
Embodiment 2
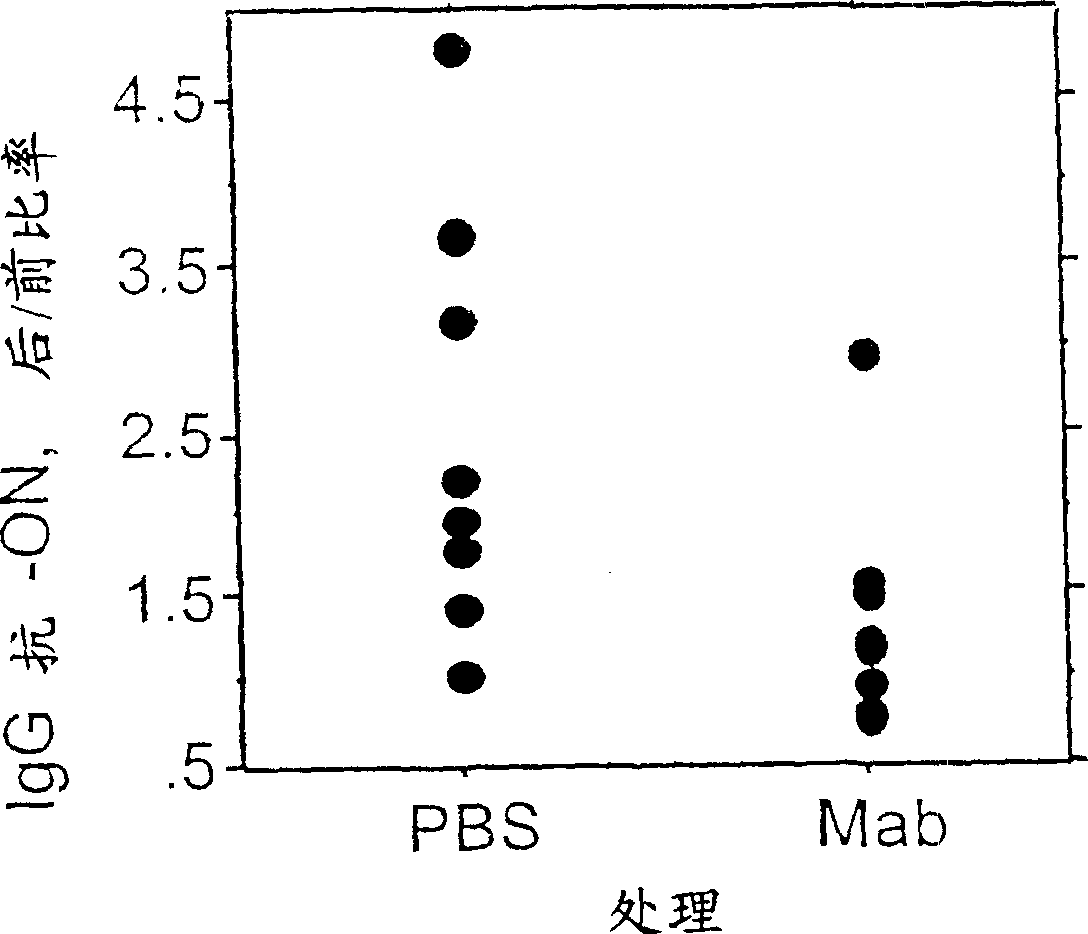
[0152] Example 2 Use of anti-CD21 antibody to reduce the level of anti-dsDNA antibody
[0153] BALB / c mice were sensitized with a model T cell-dependent antigen, which was coupled to (CA) at a ratio of 3.8 moles of oligonucleotide / mole of KLH 10 -(TG) 10 Composed of 20-mer double-stranded oligonucleotides on the keyhole: hemocyanin (KLH) (ON-KLH).
[0154] Table 2
[0155] The post / pre antibody level ratio decreased by 45% in the antibody treated group compared to the PBS treated group. Example 3A Use of anti-CD21 antibody to reduce the level of anti-dsDNA antibody in a spontaneous model of lupus
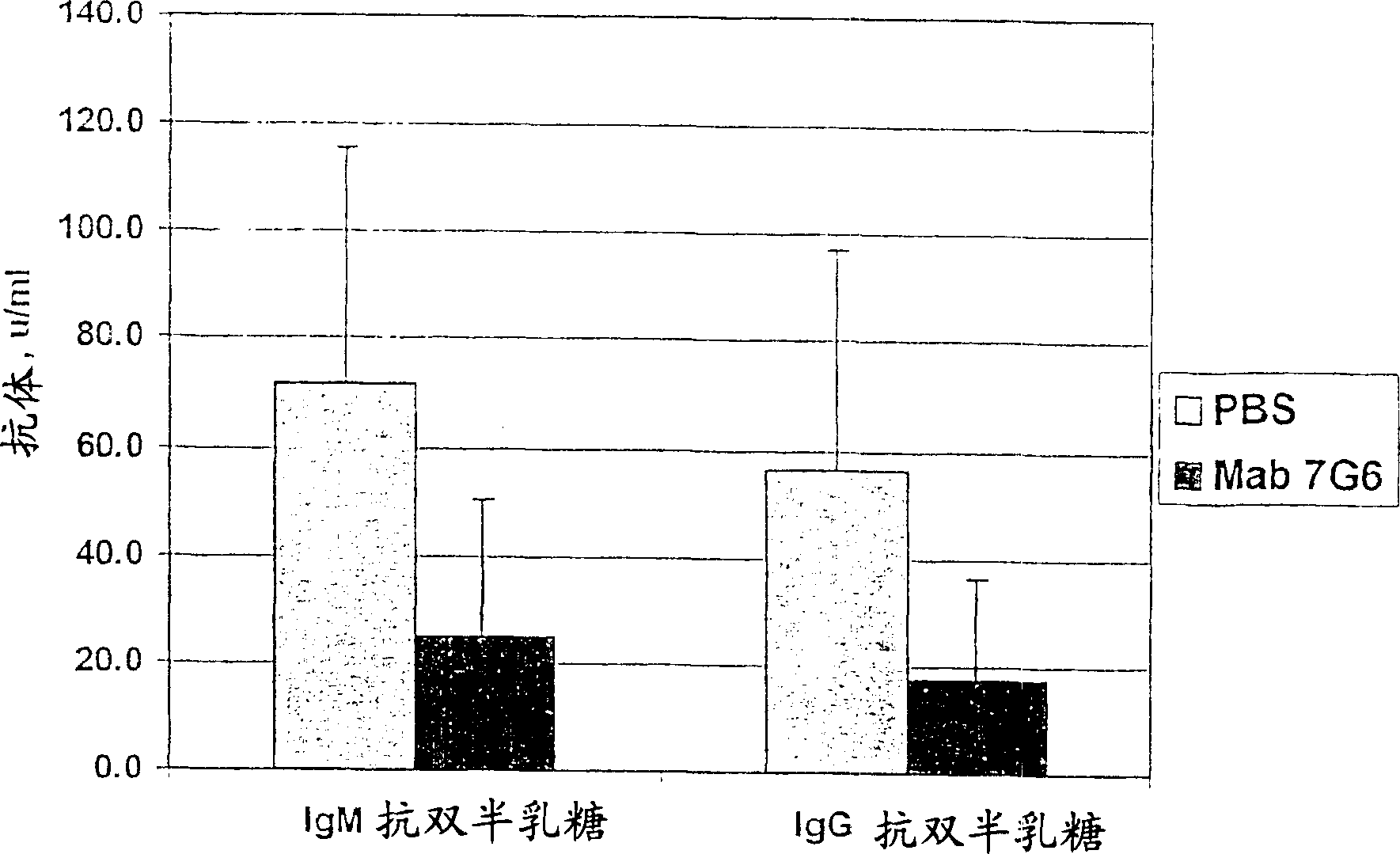
Embodiment 3A
[0155] The post / pre antibody level ratio decreased by 45% in the antibody treated group compared to the PBS treated group. Example 3A Use of anti-CD21 antibody to reduce the level of anti-dsDNA antibody in a spontaneous model of lupus
[0156] NZB / WF1(H-2 d / z ) mouse strains provide an autoimmune model to study the complex mechanisms that control the onset of autoimmunity in lupus. The animals were F1 cross progeny of NZB / B1NJ female and NZW / LacJ male mice, and they were obtained from Jackson Laboratories (Bar Harbor, Maine). The mice developed a chronic inflammatory disease of unknown origin that could be strongly influenced by genetic factors. Autoimmunity reflected by high levels of circulating anti-nuclear antibodies (including anti-double-stranded DNA antibodies); autoantibodies against RNA-protein complexes (such as RNP and Sm), ssDNA, histones, chromatin, erythrocytes, and cardiolipin; Immune complex formation and deposition in the kidney leading to proteinuria; and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com