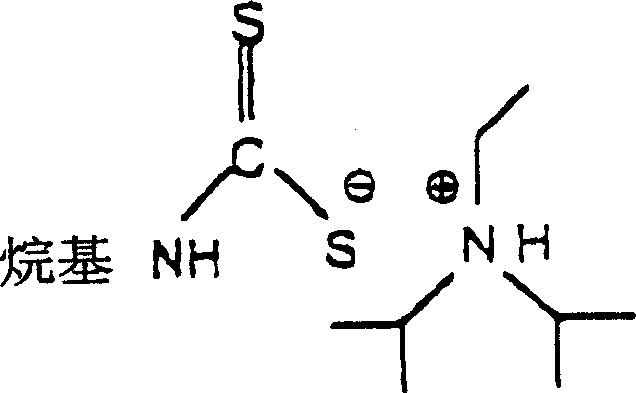
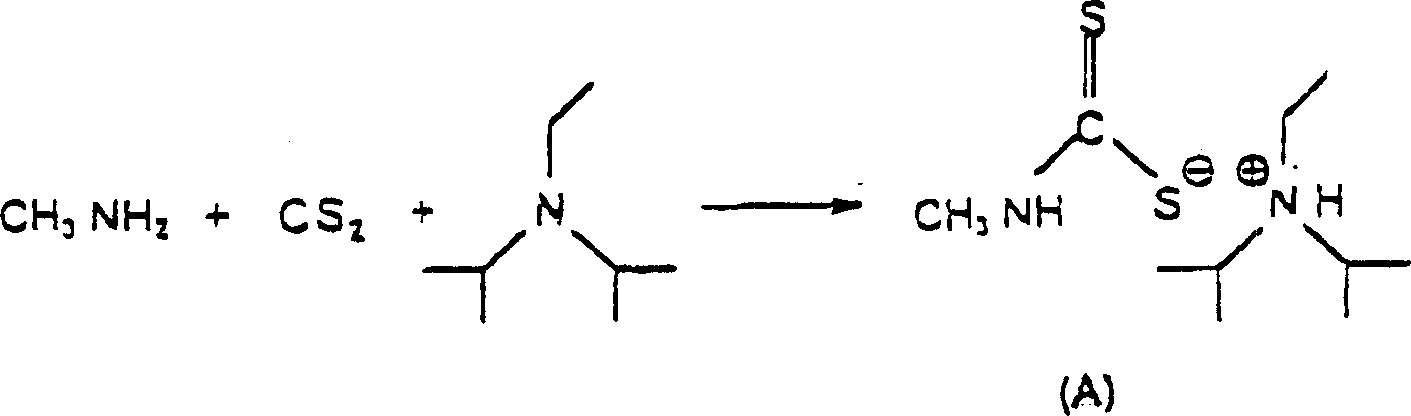
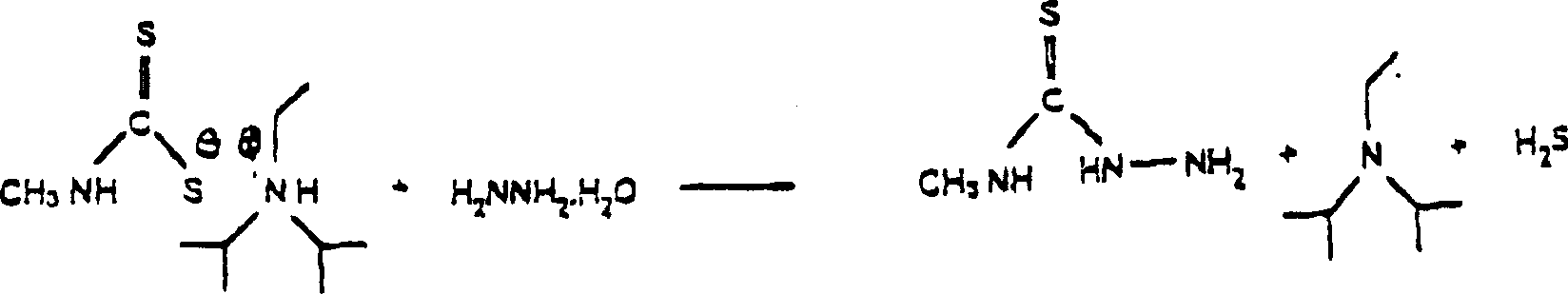Process for preparing thiosemicarbazides
A technology of thiosemicarbazide and dithiocarbamic acid, which is applied in the direction of organic chemistry and can solve the problems of not being able to prepare high-quality BTDA
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Embodiment 1 (DIPEA is excessive 10%)
[0027] Methylamine (77.5 g, 40% solution) and DIPEA (142.2 g) were charged to a 1 liter reactor equipped with a reflux condenser and stirrer. Add CS dropwise with stirring over 30 minutes 2 (76g). Drop CS 2 , the reaction temperature was kept below 30 °C by external cooling. The mixture was stirred for a further 3 hours, then the previous batch of distillate (60 g) was added. The reaction mixture was diluted with water to obtain a 24% DTC (dithiocarbamate) solution.
[0028] Hydrazine hydrate (60.1 g) was added to the DIPEA-DTC mixture. The reaction mixture was heated to 89°C and maintained at 89-92°C under reflux for 2 hours and 20 minutes. The DIPEA was distilled off (the distillation was complete when the temperature was raised above 95° C.), and the reaction mixture was cooled to 15° C. while stirring, whereupon the MTSC crystals separated from the supernatant liquid layer, yielding a yield of isolated MTSC is 73.8%. T...
Embodiment 2
[0029] Embodiment 2 (DIPEA is excessive 30%)
[0030] Methylamine (38.7 g, 40% solution) and DIPEA (82.4 g) were charged to a 0.5 liter reactor equipped with a reflux condenser and stirrer. Add CS dropwise with stirring over 30 minutes 2 (38.1g). Drop CS 2 , the reaction temperature was kept below 30 °C by external cooling. The mixture was stirred for a further 3 hours, then the previous batch of distillate (30 g) was added. The reaction mixture was diluted with water to obtain a 24% DTC (dithiocarbamate) solution.
[0031] Hydrazine hydrate (30.1 g) was added to the DIPEA-DTC mixture. The reaction mixture was heated to 92°C and held at 92°C under reflux for 2 hours and 20 minutes. DIPEA was distilled off (the distillation was complete when the temperature rose to higher than 95° C.), and the reaction mixture was cooled to 15° C. while stirring, so that the MTSC crystals were separated from the clear liquid layer, and the yield of isolated MTSC was 75.8 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com