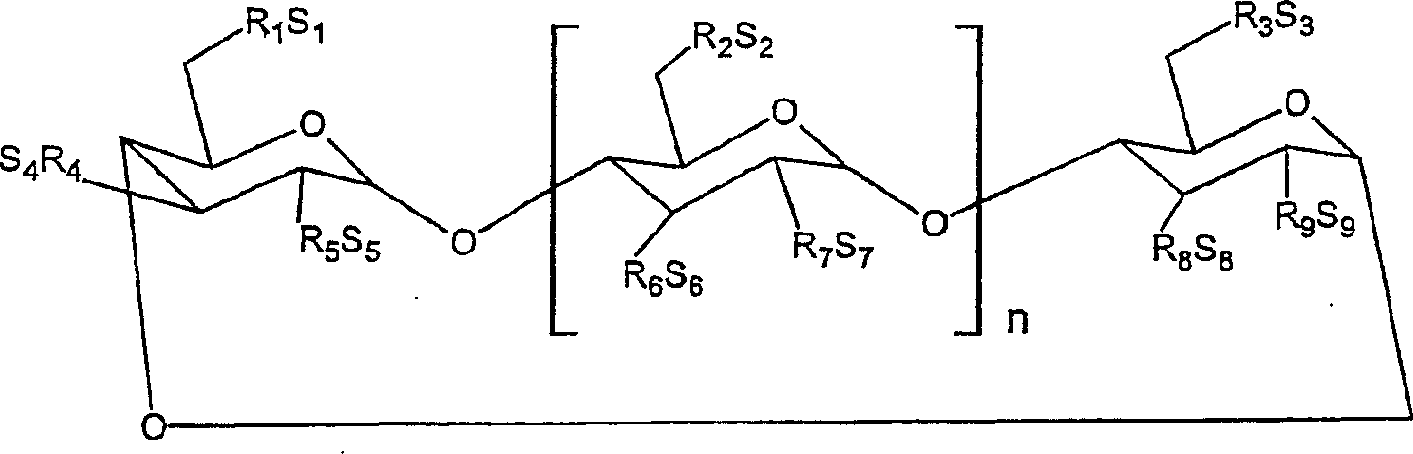
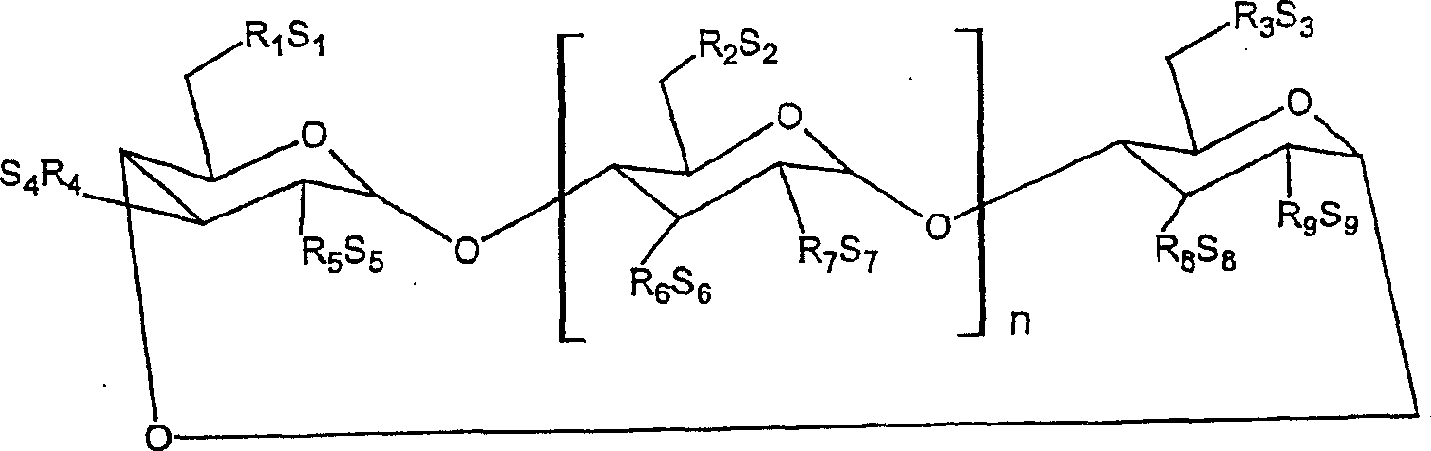
Interferon formulations
A technology of interferon and preparations, which is applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, freeze-dried transportation, etc., and can solve the problem that the coordinates cannot be obtained publicly
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0263] Materials and methods for preparing interferon gamma
[0264] Material
[0265] CHO-K1 cells (available from the American Type Culture Collection (ATCC #CCL-61 )).
[0266] HeLa cells (available from the American Type Culture Collection (ATCC #CCL-2)).
[0267] ISRE-Luc was obtained from Stratagene, La Jolla USA.
[0268] pCDNA 3.1 / hygro was obtained from Invitrogen, Carlsbad USA.
[0269] Restriction enzymes and polymerases are available from New England Biolabs Inc., Beverly, USA.
[0270] DMEM medium: Dulbecco's Modified Eagle's Medium (DMEM), 10% fetal calf serum and hygromycin B are available from Life Technologies A / S, Copenhagen, Denmark.
[0271] LucLite substrate is available from Packard Bioscience, Groningen, The Netherlands.
[0272] TopCount Luminometers are available from Packard Bioscience, Groningen, The Netherlands.
[0273] Biotinylated polyclonal anti-human interferon gamma antibody BAF285 is available from R & D Systems Inc., Minneapolis, USA.
...
Embodiment A
[0305] Example A - Determination of Surface Exposed Amino Acids
[0306] The X-ray structure used belongs to that reported by Thiel et al., Structure 8:927-936 (2000) with a third interferon gamma receptor molecule structurally non-interacting with the interferon gamma homodimer in combination with the two Interferon gamma homodimer complexed with the soluble form of the interferon gamma receptor molecule. The structure consists of an interferon gamma homodimer, with two molecules labeled A and B. For construction purposes, the interferon gamma sequence was preceded by another methionine labeled MO and the sequence was truncated at the C-terminus by 10 residues (Q133 being the last residue in the constructed polypeptide). M0 was dropped from the structure in all calculations for this example. The structures of the two interferon gamma monomers have very weak electron densities after residue 120 and the residues up to residue T126 are only models. Therefore, residues S121-...
Embodiment B
[0314] Example B - Determination of Receptor Binding Sites
[0315] ASA calculations were performed as described above to give the following residues of the interferon gamma polypeptide to have reduced ASA in at least one monomer of the complex compared to the calculated results for the isolated dimer: Q1, D2, Y4, V5, E9 , K12, G18, H19, S20, D21, V22, A23, D24, N25, G26, T27, L30, K34, K37, K108, H111, E112, I114, Q115, A118, E119.
[0316] Example C - Design for expression of interferon gamma with codons optimized for CHO cells selected expression cassette
[0317] The DNA sequence comprising the full-length cDNA encoding mature human interferon gamma with its native signal peptide, GenBank accession number X13274, was modified to favor high expression in CHO cells. The codons of the human interferon gamma nucleotide sequence were modified by forming codons that are biased in codon usage to codons commonly used by humans. Subsequently, some nucleotides in this sequen...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap