Method for purifying and preparing nucleoside triphosphate derivative
A technology for nucleoside triphosphate derivatives and nucleoside monophosphate derivatives, which is applied in the field of purification and preparation of nucleoside triphosphate derivatives, and can solve the problems of large losses, inability to completely and effectively separate raw materials and products, and low conversion rates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] Reaction formula:
[0042]
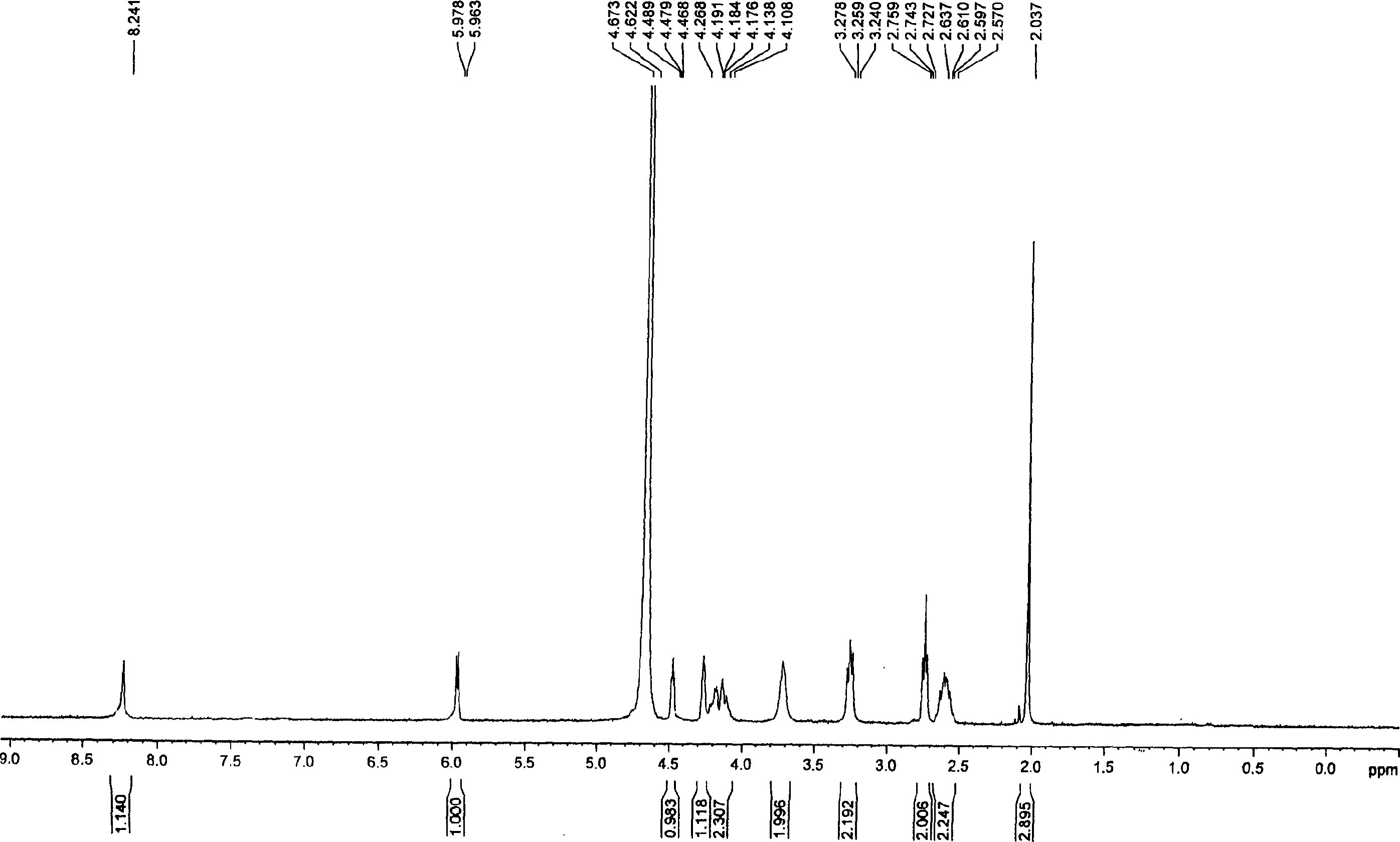
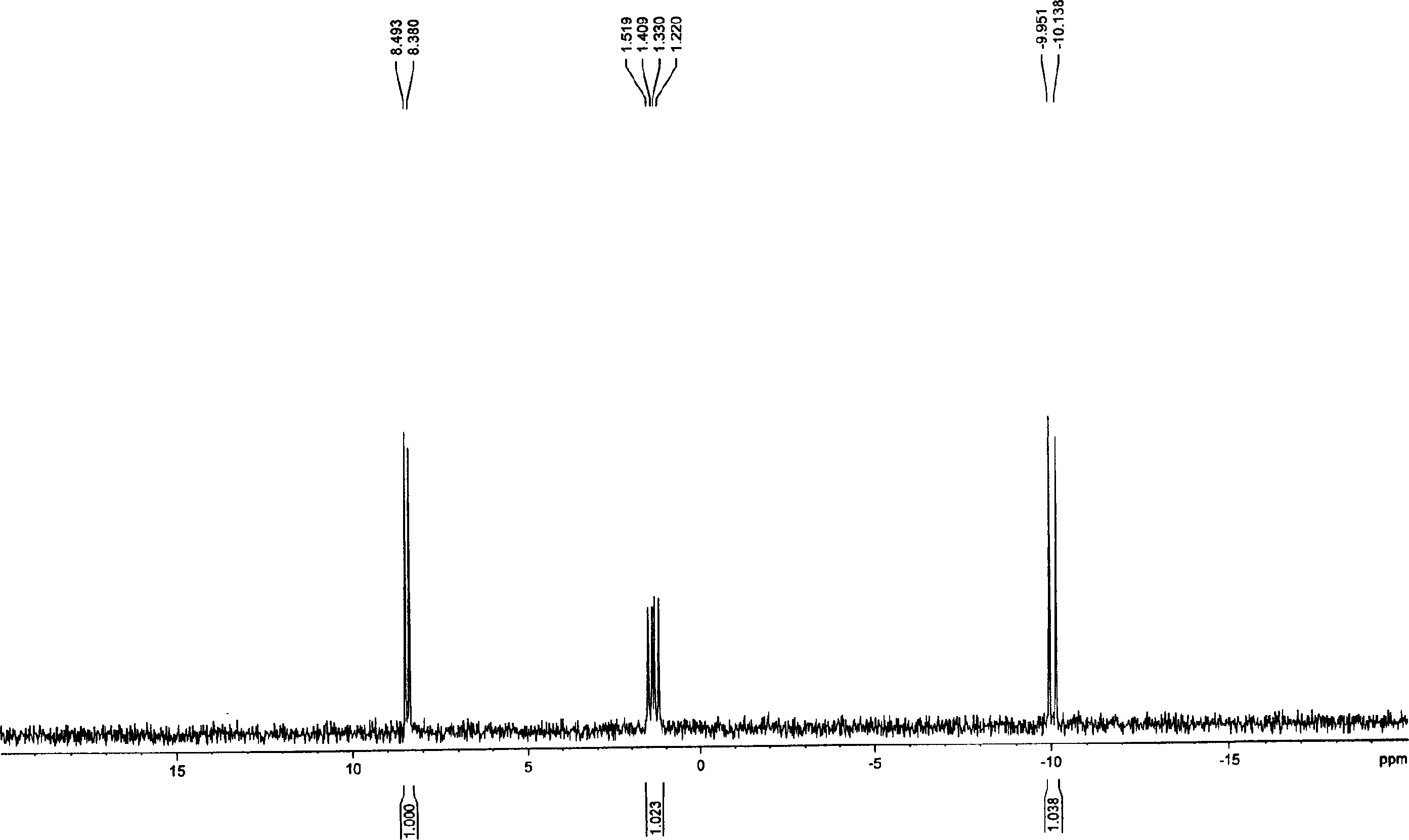
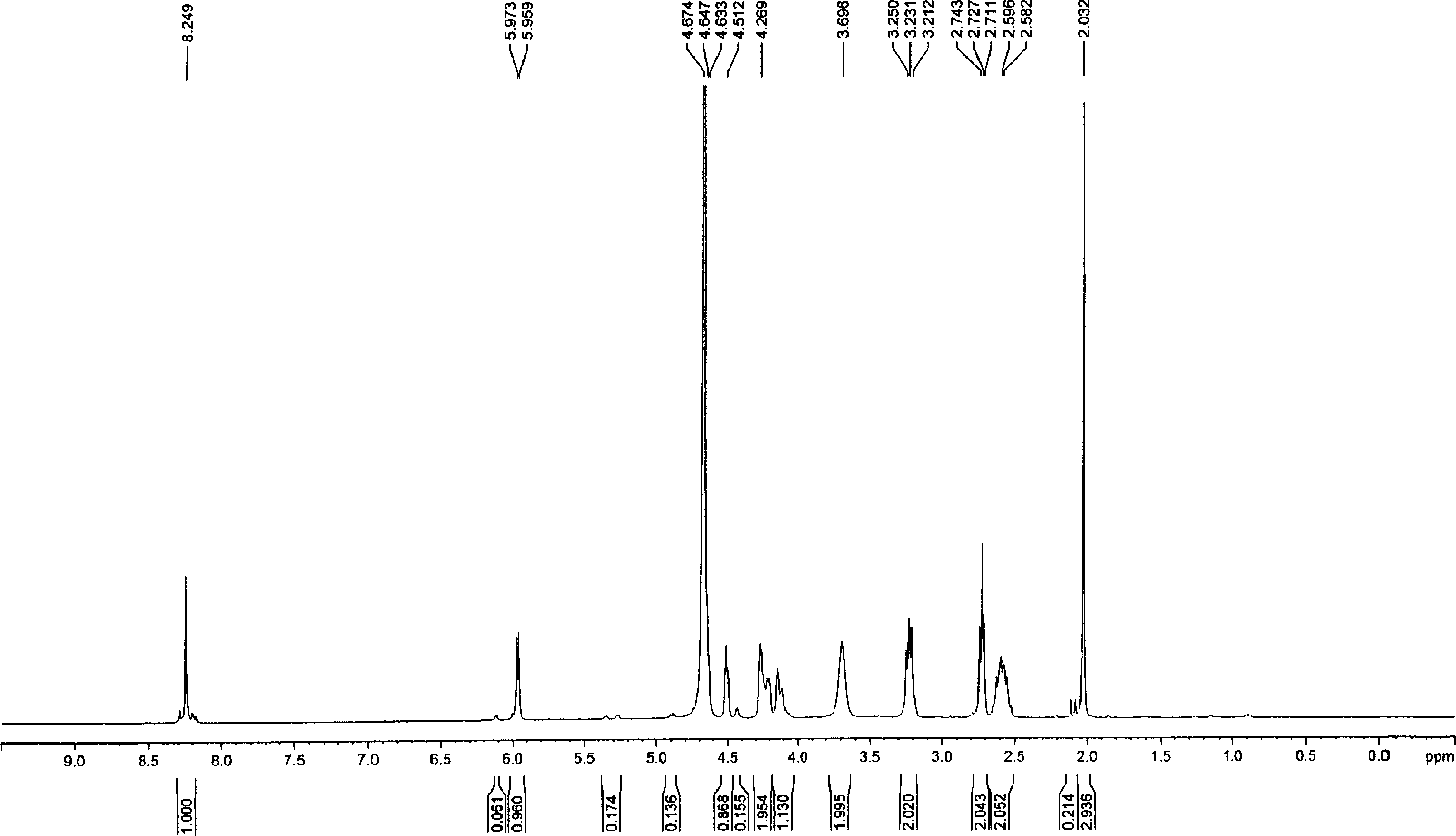
[0043] Dissolve 185 mg of tri-n-butylamine in 15 ml of pyridine, add 583 mg of nucleoside ammonium monophosphate, stir and dissolve, then dry under high vacuum at 40°C, and release the vacuum under nitrogen. Add 12 ml of anhydrous DMF, add 810 mg of CDI after dissolving, and stir overnight. Add 246 mg of methanol, after stirring for one hour, add dropwise a solution of 1392 mg of dichlorophosphoric acid and 1056 mg of tri-n-butylamine dissolved in 35 ml of DMF, stir and react at room temperature for 26 hours, filter, remove DMF under high vacuum at 60°C, add 20 milliliters of deionized water was applied to an anion exchange resin DEAD-Sephadex A-100 column for separation. Wash with 500 ml of deionized water, then wash away unreacted dichlorophosphoric acid with 250 ml of 0.1M ammonium bicarbonate and 250 ml of 0.2M ammonium bicarbonate, and finally elute with 0.3M ammonium bicarbonate. During the elution process, use UV to monitor wheth...
example 2
[0047] Dissolve 2,006 mg of nucleoside ammonium monophosphate salt that was thoroughly dried into 10 ml of anhydrous pyridine, add 665 mg of tri-n-butylamine, dissolve and vacuumize to dry up, then continue to use an oil pump to completely drain the water, and use nitrogen to release the vacuum . Add 35 ml of absolute anhydrous DMF, and add 2718 mg of CDI after dissolution. After stirring for 14 hours, 936 mg of anhydrous methanol were added. Two hours later, a solution of 4893 mg of dichlorophosphoric acid and 3694 mg of tri-n-butylamine dissolved in 120 ml of absolute anhydrous DMF was added dropwise, and the reaction was stirred at room temperature for 24 hours. Filter, drain the DMF with an oil pump, add 100 ml of deionized water, and separate with a weakly basic anion exchange resin DEAD-Sephadex A-50 column. First wash with 2000 ml of deionized water, then wash with 1000 ml of 0.1M ammonium bicarbonate and 1000 ml of 0.2M ammonium bicarbonate to wash away unreacted dic...
example 3
[0050] Reaction formula:
[0051]
[0052] Add 2.62 g of nucleoside ammonium monophosphate and 1.3 g of tri-n-butylamine into 20 ml of anhydrous pyridine, stir and dissolve, then dry under high vacuum at 40° C., and release the vacuum under nitrogen. Add 70 ml dry DMF and 5.44 g CDI. Stir overnight and add 1.88 g of anhydrous methanol. A solution of 9.8 g of dichlorophosphoric acid and 7.4 g of tri-n-butylamine dissolved in 240 ml of anhydrous DMF was added dropwise with stirring, and the reaction was stirred at room temperature for one day. Filter off the white solid, remove DMF with an oil pump, add 200 milliliters of deionized water, pour into weakly basic anion exchange resin DEAD-Sephadex A-50 column after stirring, separate, wash with 4000 milliliters of deionized water, and then wash with 2000 milliliters of deionized water respectively Wash with 0.1M ammonium bicarbonate and 2000 ml 0.2M ammonium bicarbonate, then wash with 0.3M ammonium bicarbonate. The elution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com