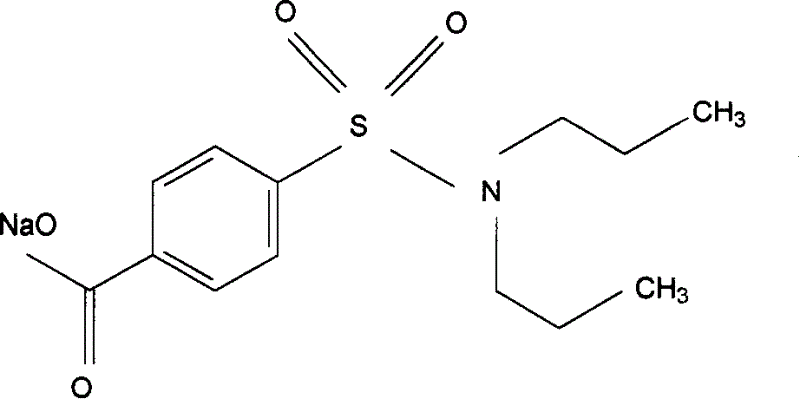
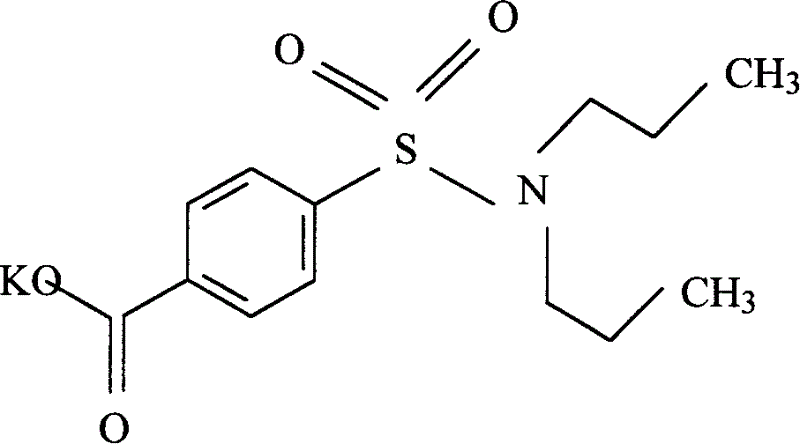
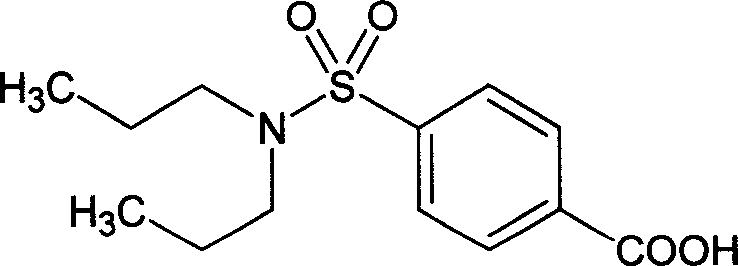
Preparation of sodium probenecid and potassium probenecid, compound injection prepared by sodium probenecid, potassium probenecid and beta-lactam antibiotics, and use thereof
A technology of probenecid sodium and probenecid potassium, which is applied in the field of powder injection and freeze-dried powder injection, can solve the problem of probenecid sodium that has not yet been seen, and achieve the effect of eliminating hemolysis and prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0347] Example 1: Dissolve 22 kilograms of sodium hydroxide in 200 liters of purified water, add 142 kilograms of probenecid after all the dissolution, heat to 60° C., adjust the pH to above 7.5, and keep stirring until the pH value is basically constant. Under the condition of 60°C, it is sterilized by filtration through a 0.45 μm filter membrane, and then finely filtered through a 0.22 μm filter membrane to remove pyrogens, and dried under sterile conditions to obtain the raw material of probenecid sodium that meets the standards for human drug injections. White or off-white crystalline powder.
[0348] Probenecid sodium and penicillin G sodium are mixed under aseptic conditions at a weight ratio of 1:3, subpackaged under aseptic conditions, and finally made into a powder injection.
Embodiment 2
[0349] Example 2: Dissolve 28 kg of sodium hydroxide in 280 liters of purified water, add 143 kg of probenecid after all the dissolution, heat to 65°C, adjust the pH to above 8, and keep stirring until the pH value is basically constant. Under the condition of 65°C, it is sterilized by filtration through a 0.45 μm filter membrane, and then finely filtered through a 0.22 μm filter membrane to remove pyrogens, and dried under sterile conditions to obtain the raw material of probenecid sodium that meets the standards for human drug injections. White or off-white crystalline powder.
[0350] Probenecid sodium and ampicillin sodium are mixed under aseptic conditions at a weight ratio of 1:2.8, subpackaged under aseptic conditions, and finally made into a powder injection.
Embodiment 3
[0351] Example 3: Dissolve 40 kg of sodium hydroxide in 300 liters of purified water, add 150 kg of probenecid after all dissolved, heat to 70°C, adjust pH to 10, and keep stirring until the pH value is basically constant. Under the condition of 70°C, it is sterilized by filtration through a 0.45 μm filter membrane, then finely filtered through a 0.22 μm filter membrane to remove pyrogens, and dried under sterile conditions to obtain the raw material of probenecid sodium that meets the standards for human drug injections. White or off-white crystalline powder.
[0352] Probenecid sodium and cefazolin sodium are mixed under aseptic conditions at a weight ratio of 1:2, subpackaged under aseptic conditions, and finally made into a powder injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com