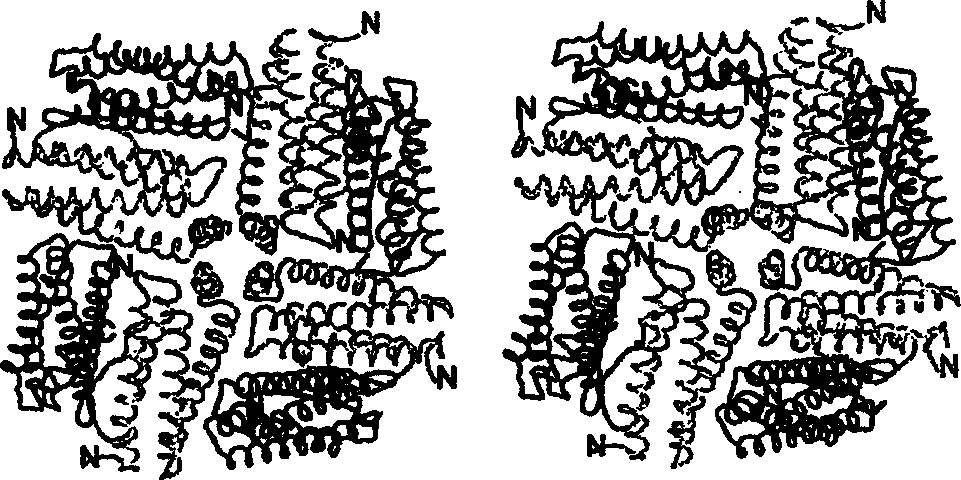
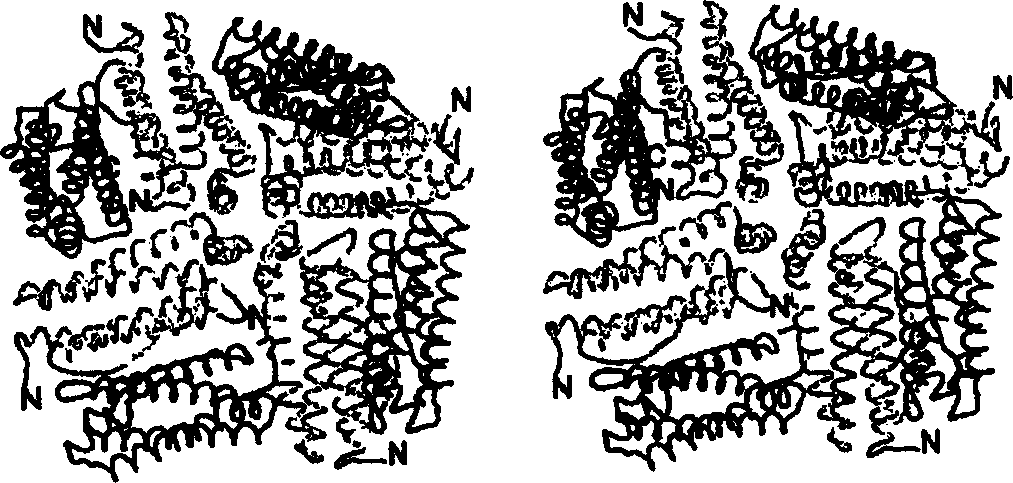
Ferritin fusion proteins for use in vaccines and other applications
A fusion protein and ferritin technology, applied in the field of fusion ferritin, can solve problems such as strengthening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0073] Example 1 Fusion protein expressed in granules:
[0074] Human hemoglobin protein α chain is connected to the C-terminus of human ferritin through a single glycine linker recombinant fusion protein
[0075] Particle abbreviation: (F L.G.Hα).
[0076] Molecular cloning: the recombinant protein fused with human hemoglobin α subunit and human ferritin L chain was expressed in Escherichia coli. The full-length cDNA of the hemoglobin α chain was connected to the C-terminus of the ferritin light chain gene by a linker consisting of a glycine ( image 3 ).
[0077] Protein expression and purification: The coding sequence of ferritin / hemoglobin was verified by DNA sequencing. The recombinant transformed protein was expressed in bacteria BL21(DE3). When the transformed bacteria grew to an OD value of 1.0 (600nm), 1mMIPTG was added to activate the T7 promoter, and the expression was induced at 30°C. After expression, the cells were resuspended in B-PER buffer and sonica...
example 2
[0084] Example 2 Fusion protein expressed in granules:
[0085] The silver-enriched polypeptide is bound to human ferritin L chain C via a linker containing 2 glycines recombinant fusion protein
[0086] Particle abbreviation: (F L .GG.Ag4), AG4 refers to NPSSLFRYLPSD (serial number: 1).
[0087] As a metal-extracting polypeptide linked to ferritin, the particle was shown to be in the correct conformation by the following observations:
[0088] 1) The purified expression product eluted by size exclusion gel chromatography has the same retention effect as natural recombinant ferritin (ferritin molecular weight: 408K).
[0089] 2) Figure 7 and protein light scattering experiments in Table 3 show that individual dispersed proteins have a diameter of about 180 angstroms;
[0090] 3) The particle characteristics of silver condensing peptide have been clarified;
[0091] Meas.#
[0092] Table 3 (F L .GG.Ag4) data sheet. The results were detected using a Protein...
example 3
[0093] Example 3 extragranular fusion protein
[0094] HIV Tat protein (84 amino acids) through a linker containing 6 glycines Linked to the N-terminus to form a recombinant protein.
[0095] Particle abbreviation: (Tat.6G.F L )
[0096] in:
[0097] HIV Tat protein sequence:
[0098] MEPVDPRLEP WKHPGSQPKT ACTNCYCKKC CFHCQVCFIT
[0099] KALGISYGRK KRRQRRRAHQ NSQTHQASLS KQPTSQPRGD
[0100] PTGPKE - (serial number: 2)
[0101] Glycine linker sequence:
[0102] GGGGGG (serial number: 3)
[0103] Human ferritin L chain sequence:
[0104] MSSQIRQNYS TDVEAAVNSL VNLYLQASYT YLSLGFYFDR DDVALEGVSH
[0105] FFRELAEEKR EGYERLLKMQ NQRGGRALFQ DIKKPAEDEW GKTPDAMKAA
[0106] MALEKKLNQA LLDLHALGSA RTDPHLCDFL ETHFLDEEVK LIKKMGDHLT
[0107] NLHRLGGPEA GLGEYLFERL TLKHD (Serial Number: 4)
[0108] Molecular cloning: expression of wild-type HIV-1 Tat fused with human ferritin L-chain recombinant protein in Escherichia coli. Using the PCR method, the full-length cDNA of the Tat prot...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com