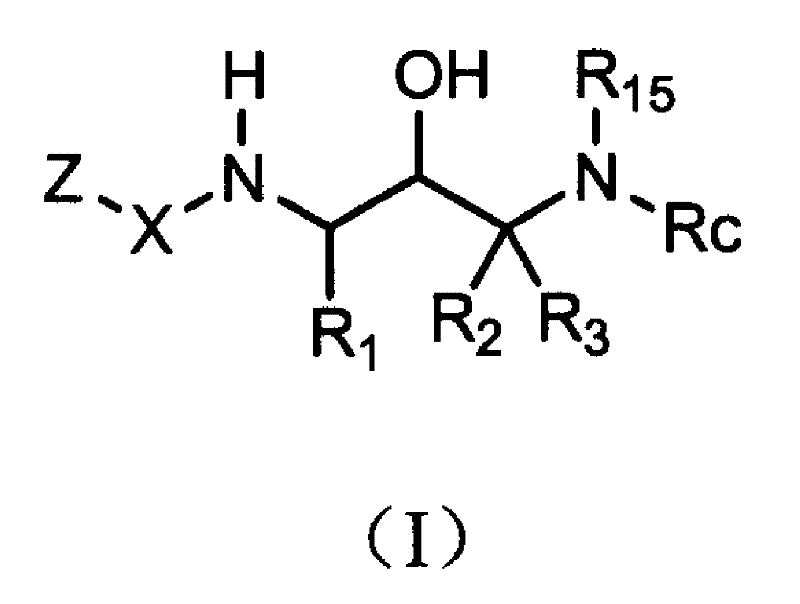
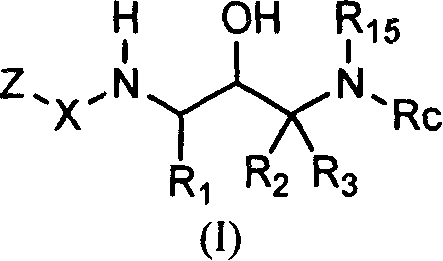
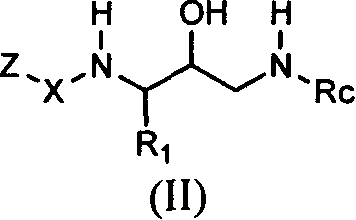
Acetyl 2-hydroxy-1, 3-diaminoalkanes
An alkyl, radical technology, applied in the field of acetyl 2-hydroxy-1, which can solve the problem of effective treatment methods that do not suspend, prevent or reverse the progression of Alzheimer's disease, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0854] Embodiment 1: the preparation of 7-bromo-1-tetralinone
[0855]
[0856] 7-Bromo-1-tetralinone was prepared following the procedure described in Cornelius, L.A.M.; Combs, D.W. Synthetic Communications 1994, 24, 2777-2788. The above isomers were separated using flash chromatography on silica gel (BiotageFlash 75, eluent solvent 20 / 1 hexane:MTBE) to yield 5-bromo-1-tetralinone (11.59 g, 51%) and 7-bromo-1 - Tetralinone (9.45 g, 42%).
Embodiment 2
[0858] Embodiment 2: the preparation of (R)-7-ethyltetralin-1-alcohol
[0859]
[0860] See generally: Jones, T.K.; Mohan, J.J.; Xavier, L.C.; Blacklock, T.J.; Mathre, D.J.; Sohar, P.; , 56, 763-769. More specifically, 7-ethyl-1-tetralinone (2.29 g, 13.1 mmol) was placed in a 100 mL round bottom flask and dissolved in anhydrous THF (40 mL). Activated 4mm molecular sieves were added and the mixture was aged for 2 hours before cannula transfer to a 250 mL three necked round bottom flask equipped with a dropping funnel, thermometer and nitrogen inlet. The solution was cooled to -25°C and 1M (S)-tetrahydro-1-methyl-3,3-diphenyl-1H,3H-pyrollo[1 ,2-c][1,3,2]oxazaborole. The source of oxazoborole is Aldrich, cat. No. 45, 770-1, "(S)-2-Methyl-CBS-oxazaborolidine". The use of S-promoters generates R-alcohols. According to the aforementioned references, the use of 5 mol% oxazaborolidine catalyst gave comparable results.
[0861] A solution of borane-dimethylsulfide (0.70 g, 0.8...
Embodiment 3
[0862] Example 3: (S)-7-ethyl-1,2,3,4-tetrahydro-1-naphthylamine hydrochloride
[0863]
[0864] See generally: Rover, S.; Adam. G..; Cesura, A.M.; Galley, G.; Jenck, F.; -1338. Their authors reported a slight decrease in yield by partial formation of dihydronaphthalene through removal of the hydroxyl moiety.
[0865] More specifically, a solution of (R)-7-ethyltetralin-1-ol (1.77 g, 10.1 mmol) in toluene (25 mL) was cooled in an ice bath and treated with diphenylphosphoryl azide ( DPPA, 3.3 g, 2.7 mL, 12 mmol). A solution of 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU, 1.8 g, 1.8 mL, 12 mmol) in toluene (8 mL) was added over 20 minutes, and the mixture was heated at 0° C. Stir for 2 hours and 16 hours at room temperature. The mixture was filtered through a pad of silica gel (elution 6:1 hexane / ethyl acetate) to remove the precipitate and the volatiles were removed in vacuo to give crude S-azide as an oily residue. This material was used directly in the next step without f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com