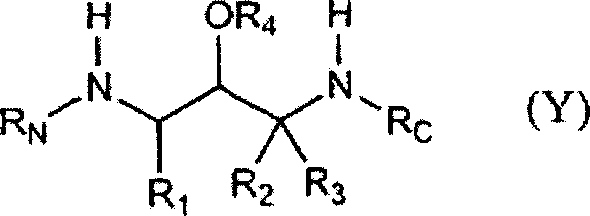
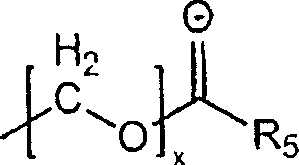
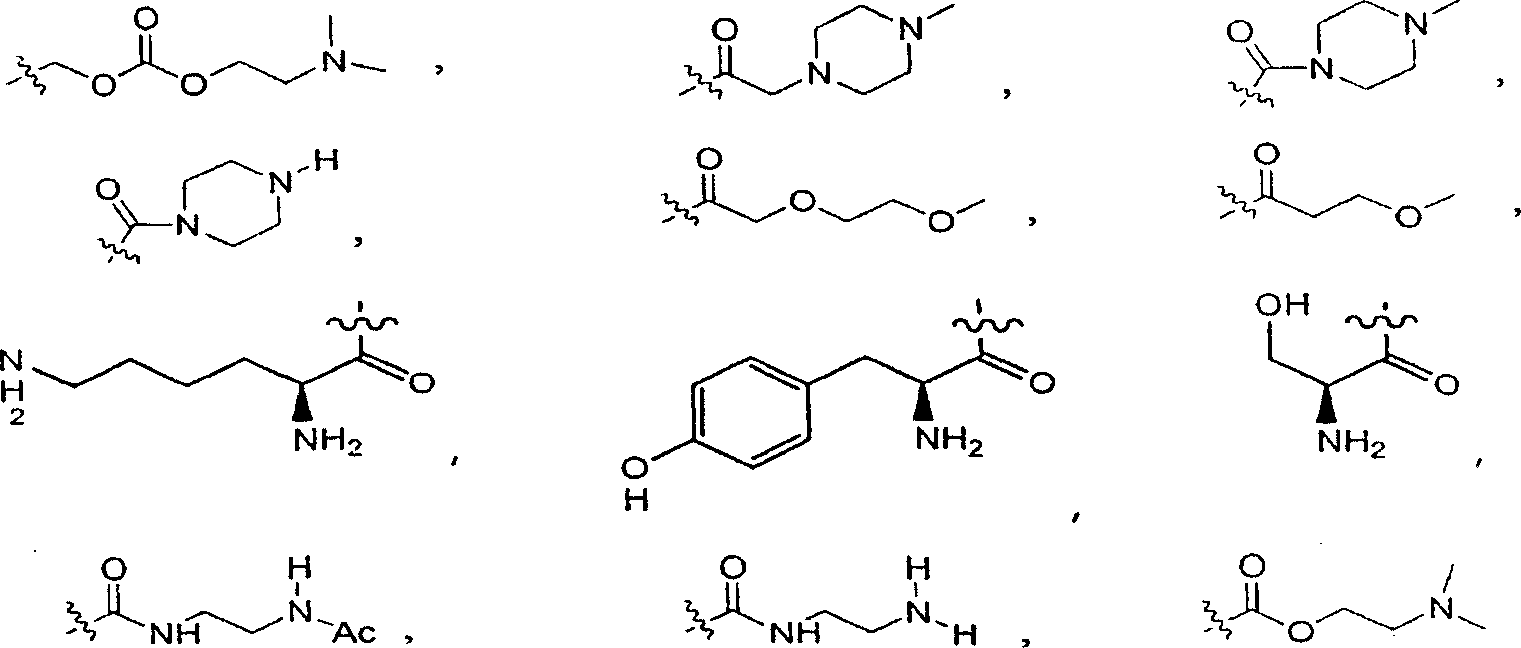
Substituted hydroxyethylamines
一种取代基、烷基的技术,应用在胺化合物的前体药物领域,能够解决没有中止、预防或逆转阿尔茨海默氏症深化的有效治疗方法等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0782]Panel A illustrates a general method for preparing compound (Y) used in the present invention. The anti-Alzheimer's compound (Y) of the present invention is prepared using the corresponding amino acid (I) as a raw material. The amino acid (I) is known to those skilled in the art, or can be easily prepared from known compounds by methods known to those skilled in the art. Compound (Y) of the present invention has at least two enantiocenters, which give rise to four enantiomers. The first of these enantiocenters is derived from the starting amino acid (I). It is preferred to obtain or prepare the desired enantiomer (S) commercially rather than to produce an enantiomerically non-unitary mixture and then have to isolate the desired enantiomer (S). The method preferably uses (S)-amino acid (I) having a single enantiostructural structure and the same configuration as the product compound (Y) as a starting material.
[0783] More preferably, R 1 Yes-(CH 2 ) 0-1 -(R 1-芳基 ...
Embodiment 1
[1064] Example 1(1S)-3-bromo(3,5-difluorobenzyl)-2-oxopropylcarbamate tert-butyl ester (III)
[1065] N-Methyl-morpholine (5.83 mL, 53 mmol, 1.05 eq.) was added to (2S)-2-[(tert-butoxycarbonyl)amino]-3-(3 , 5-difluorophenyl)propionic acid (II, 15 g, 50 mmol), and the reaction was cooled to -78°C. Isobutyl chloroformate (6.87 mL, 53 mmol, 1.05 eq.) was added rapidly. The cooling bath was then removed and the mixture was stirred for 1 hour. The reaction was monitored by TLC to ensure completion, then the mixture was filtered and washed with anhydrous THF (50 mL) and kept cold at -20°C in the filter flask.
[1066] A 500-mL graduated cylinder containing diethyl ether (200 mL) and aqueous potassium hydroxide (40%, 60 mL) was placed in an ice-salt bath. 1-Methyl-3-nitro-1-nitrosoguanidine (5.6 g, 106 mmol, 2.1 eq.) was added slowly while stirring, keeping the temperature below 0°C. The mixture turned yellow and continued to foam for 10 minutes. Stirring was stopped, the layers...
Embodiment 2
[1067] Example 2 (1S, 2S)-3-bromo-1-(3,5-difluorobenzyl)-2-hydroxypropylcarbamate tert-butyl ester (IV)
[1068] Add sodium borohydride (1.32 g, 34.9 mmol, 1.1 eq.) to (1 S)-3-bromo(3,5-difluorobenzyl)- In tert-butyl 2-oxopropylcarbamate (III, Example 1, 12 g, 31.75 mmol). The reaction mixture was stirred for 0.5 h and passed by TLC (ethyl acetate / hexane, 20 / 80; Rf = 0.2). The mixture was quenched with water (10 mL), and the solvent was removed by heating under reduced pressure (not exceeding 30° C.) to dryness. The solid was partitioned between dichloromethane and water, washed with brine and dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure to obtain the title compound, TLC (ethyl acetate / hexane, 20 / 80) R f =0.2; MS(MH + ) = 381.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com