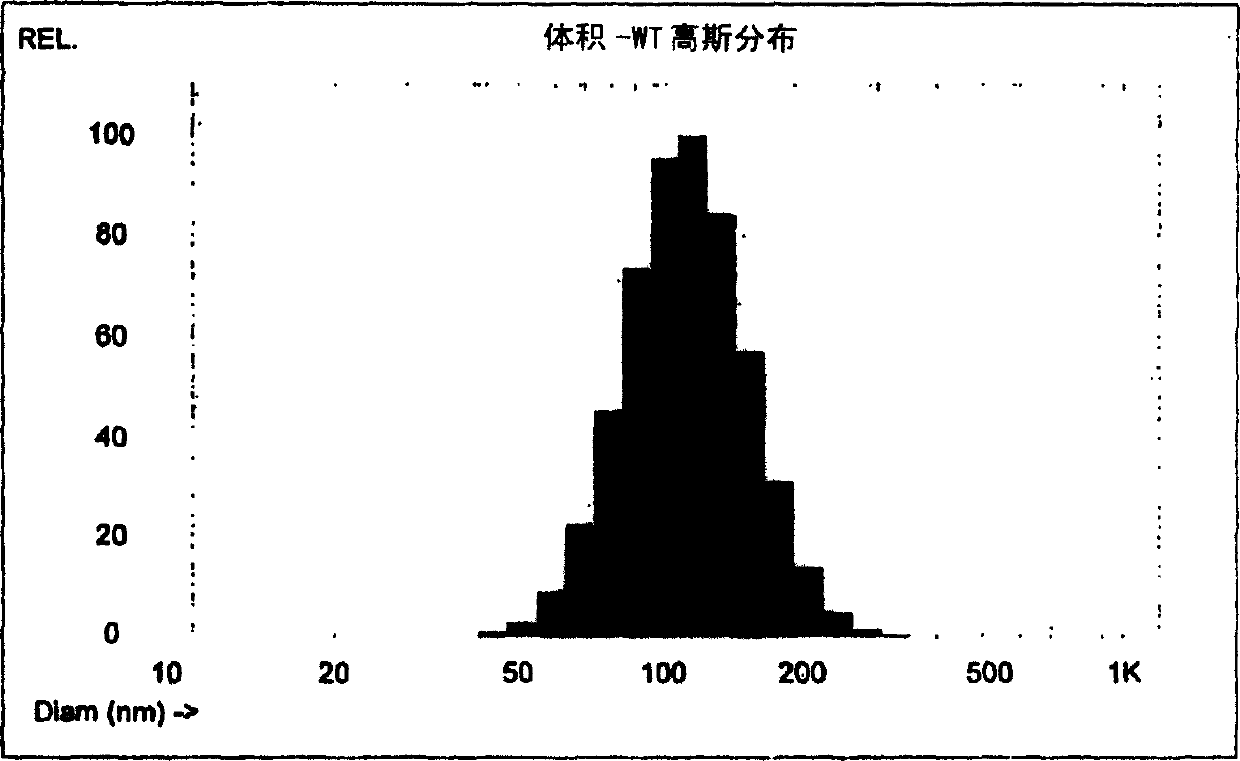
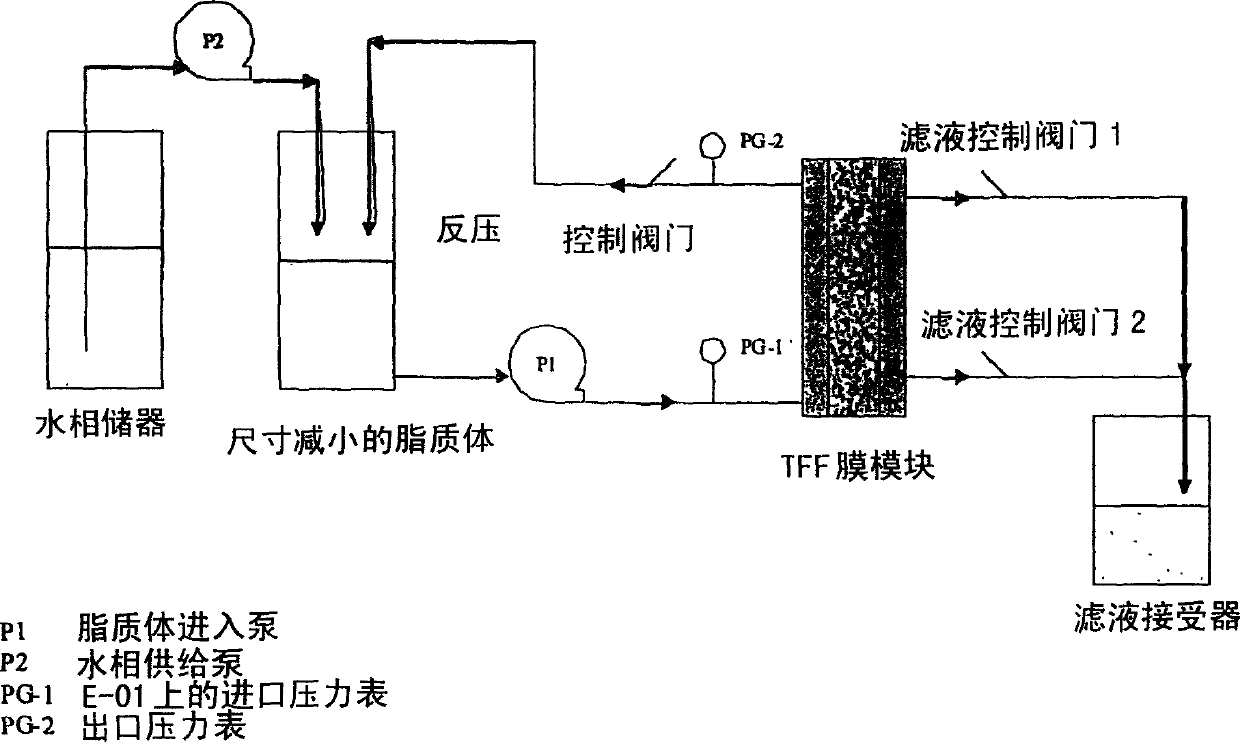
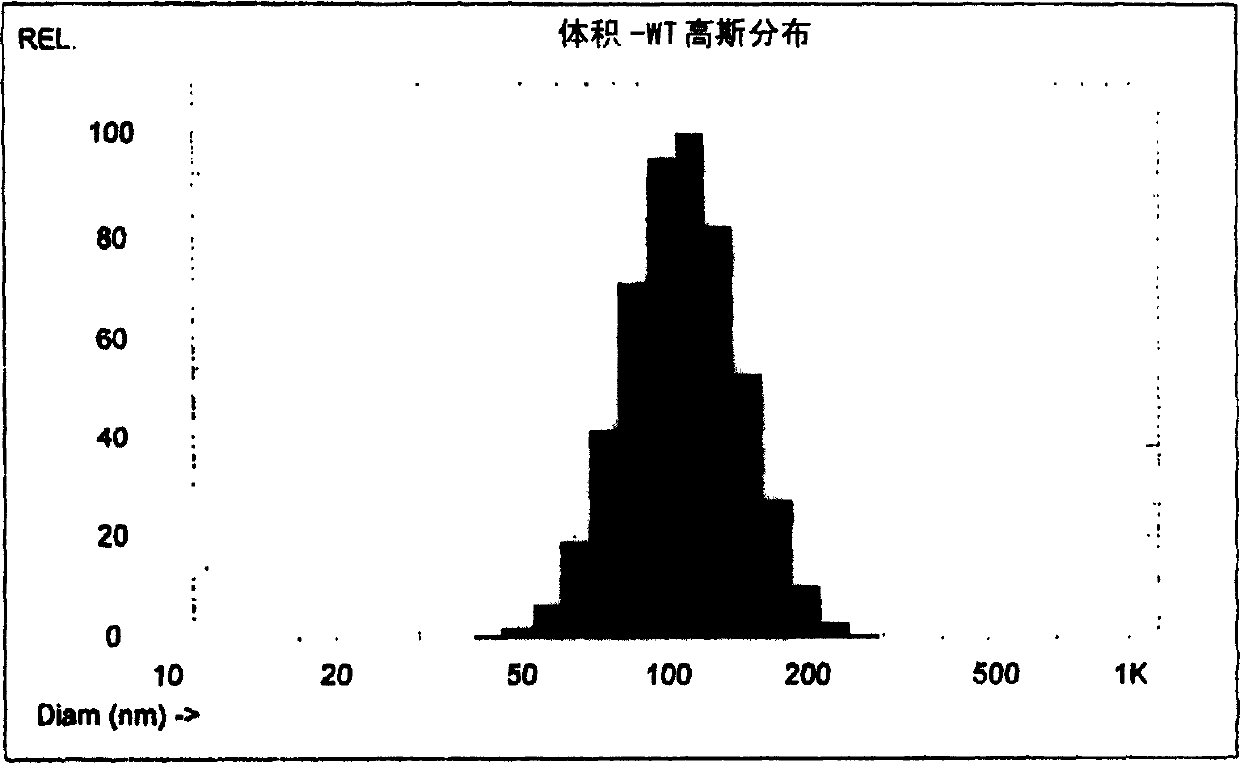
Manufacturing process for liposomal preparations
A liposome preparation, lipid technology, applied in the field of liposome preparations, preparation of liposome preparations, can solve problems such as insufficient supply
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention also provides liposomal formulations produced by the methods of preparation as described herein and methods of use of these formulations. Liposome formulations of the invention can generally be formulated for administration to human or animal patients. For these applications, the formulations of the invention may comprise, in addition to the liposomal formulation of the active agent, nontoxic, inert pharmaceutically suitable excipients. Pharmaceutically suitable excipients include all types of solid, semi-solid or liquid diluents, fillers and formulation auxiliaries. Tablets, lozenges, capsules, pills, granules, suppositories, solutions, suspensions and emulsions, pastes, ointments, gels, creams, lotions, powders or sprays may be suitable pharmaceutical preparations. Suppositories may contain, in addition to liposomal active agents, suitable water-soluble or water-insoluble excipients. Suitable excipients are those in which the liposomal active a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| surface area | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com