Production of pivalic acid
A pivalic acid and pressure technology, which is applied in the field of producing pivalic acid by synthetic methods, can solve the problems of affecting market competitiveness, high cost, and high cost of pivalic acid, so as to reduce energy consumption, concentration, and production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
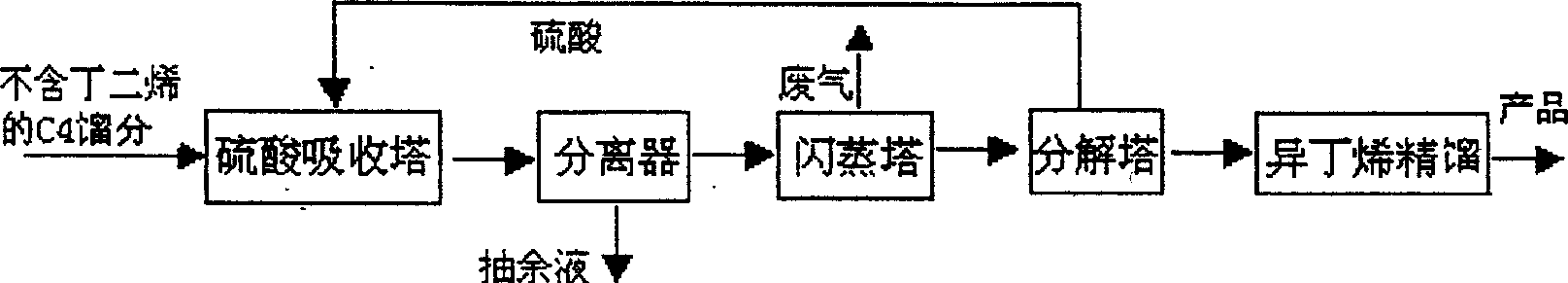
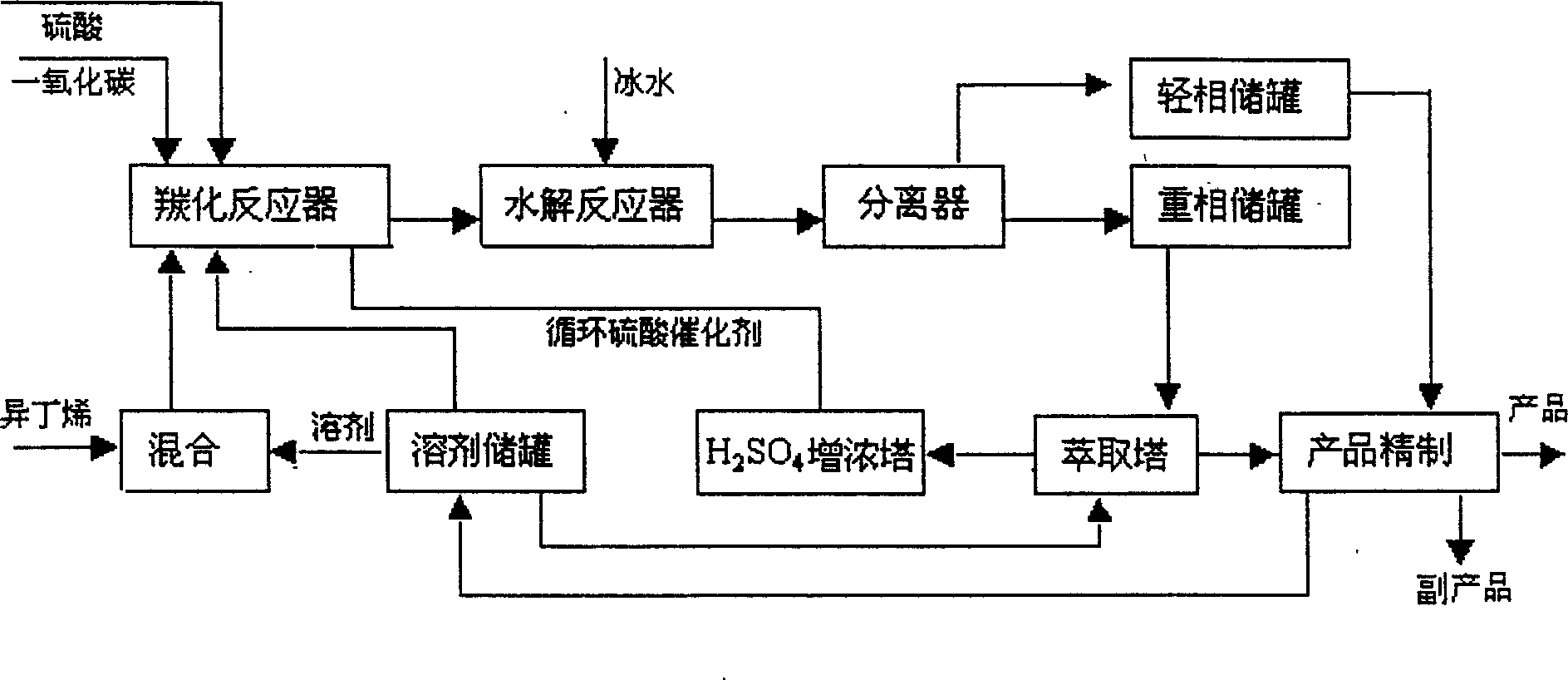
[0031] With 1.65 tons of butadiene-free carbon four cuts (isobutene 45%, isobutane 3%, 1-butene 25%, n-butane 11%, trans-2-butene 9%, cis-2-butene 7 % and 1,3-butadiene <0.5%) and 2.7 tons of 50% sulfuric acid as raw materials, the reaction temperature is 15-40 ° C and the pressure is 0.12-0.45Mpa, respectively enter the sulfuric acid absorption tower for esterification. The reaction mixture (tert-butyl sulfate, tert-butanol and raffinate containing isobutylene) enters the separator for separation, and the lower layer mixture (tert-butyl sulfate, tert-butanol) enters the flash column for flashing to remove A small amount of hydrocarbons, the mixture obtained (the total content of tert-butyl sulfate and tert-butanol is more than 99% after analysis), enters the mixer and mixes with chloroform from the solvent storage tank, and the mixed solution enters the carbonylation reactor , add 4.3 tons of 85% sulfuric acid in the carbonylation reactor simultaneously, carry out carbonylati...
example 2
[0033] With 1.65 tons of butadiene-free carbon four cuts (isobutene 45%, isobutane 3%, 1-butene 25%, n-butane 11%, trans-2-butene 9%, cis-2-butene 7 % and 1,3-butadiene <0.5%) and 3.0 tons of 50% sulfuric acid as raw materials, the reaction temperature is 15-40°C and the pressure is 0.12-0.45Mpa, respectively enter the sulfuric acid absorption tower for esterification. The reaction mixture (tert-butyl sulfate, tert-butanol and raffinate containing isobutylene) enters the separator for separation, and the lower layer mixture (tert-butyl sulfate, tert-butanol) enters the flash column for flashing to remove A trace amount of hydrocarbons, the mixture obtained (the total content of tert-butyl sulfate and tert-butanol reaches more than 99% after analysis) enters the mixer and mixes with the chloroform from the solvent storage tank, and the mixed solution enters the carbonylation reactor, Add 4.3 tons of 85% sulfuric acid in the carbonylation reactor simultaneously, carry out carbon...
example 3
[0035]With 1.65 tons of butadiene-free carbon four cuts (isobutene 45%, isobutane 3%, 1-butene 25%, n-butane 11%, trans-2-butene 9%, cis-2-butene 7 % and 1,3-butadiene <0.5%) and 2.7 tons of 50% sulfuric acid as raw materials, the reaction temperature is 10-15°C and the pressure is 0.1-0.12Mpa, respectively enter the sulfuric acid absorption tower for esterification. The reaction mixture (tert-butyl sulfate, tert-butanol and raffinate containing isobutylene) enters the separator for separation, and the lower layer mixture (tert-butyl sulfate, tert-butanol) enters the flash column for flashing to remove A trace amount of hydrocarbons, the mixture obtained (the total content of tert-butyl sulfate and tert-butanol reaches more than 99% after analysis) enters the mixer and mixes with the chloroform from the solvent storage tank, and the mixed solution enters the carbonylation reactor, Add 4.3 tons of 85% sulfuric acid in the carbonylation reactor simultaneously, carry out carbonyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com