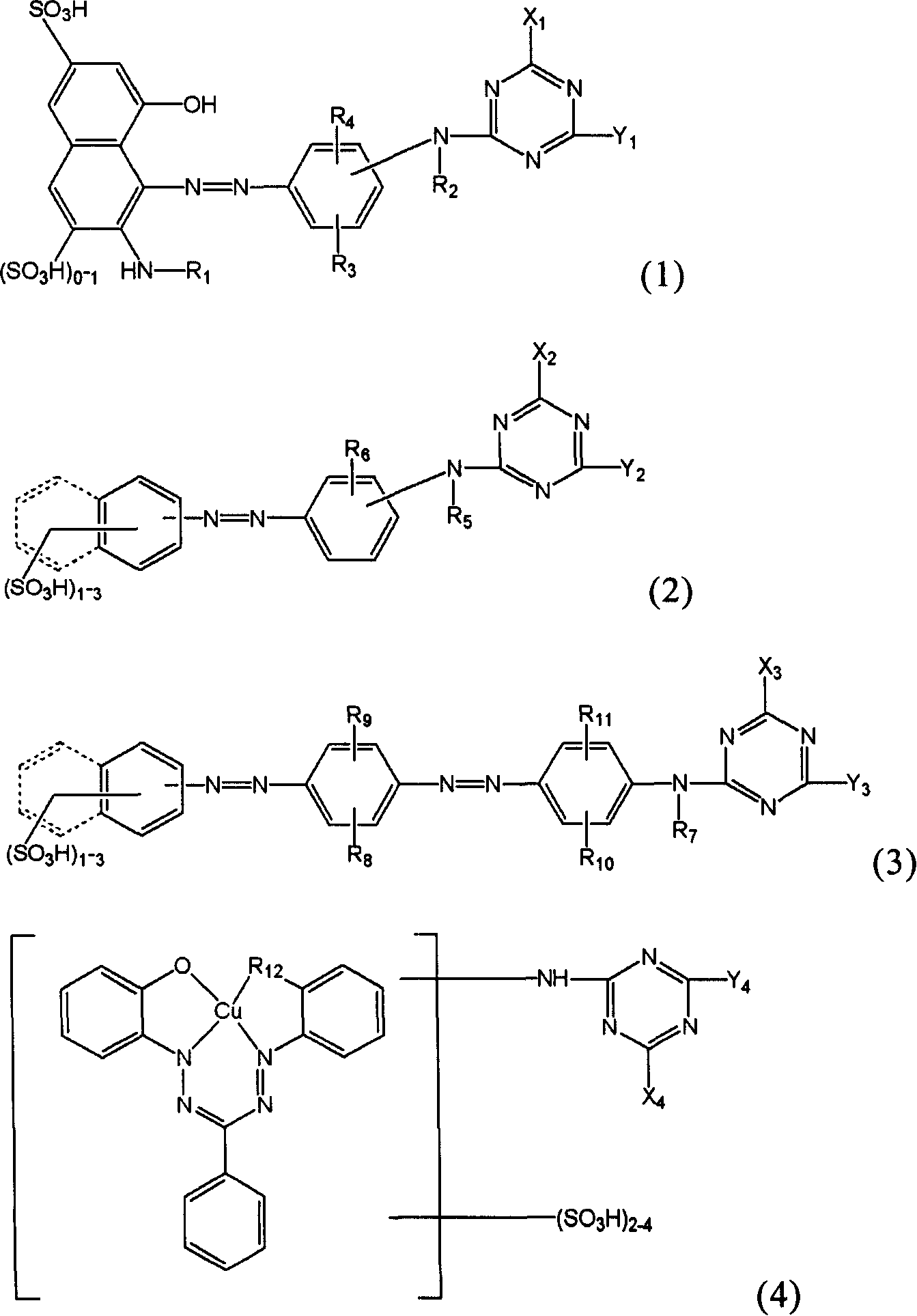
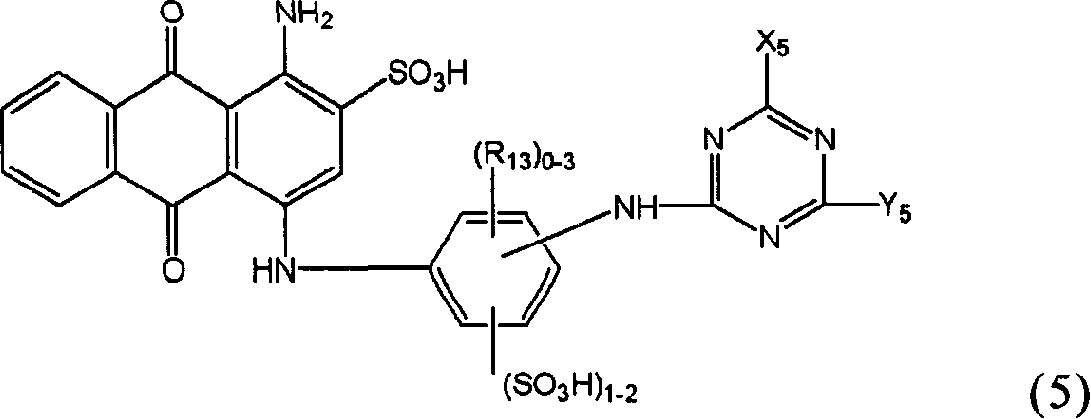
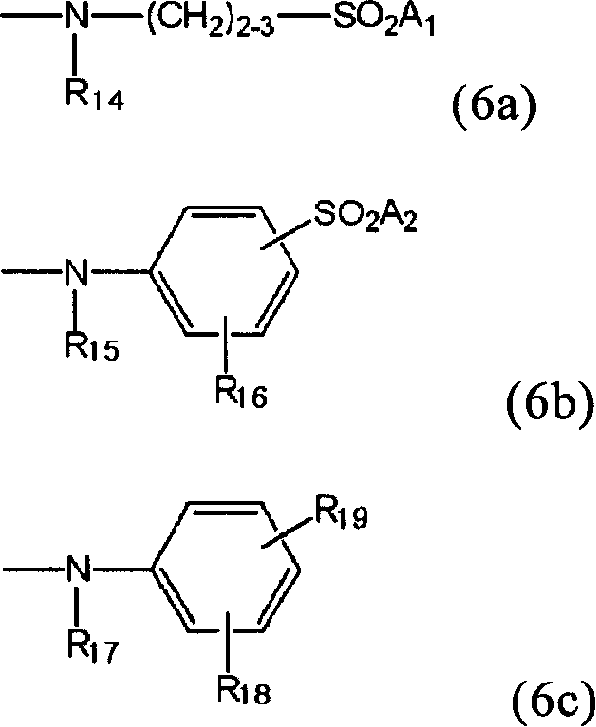
Novel reactive dye composition with three-color combination
A technology of dye composition and reactive red dye, applied in the field of new reactive dye composition, to achieve the effects of balanced physical properties, high light fastness, excellent adsorption and color fixing ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 51.8 g of the compound of formula 15 shown below was dissolved in 500 g of water, and 100 g of ice was added to the resulting solution, thereby cooling the solution. 19.0 g of cyanuric chloride was added to the solution, and the mixture was stirred and reacted at 5° C. and pH 5 for 2 hours. Then, 22.5 g of 2-aminoethyl-2'-sulfatoethylsulfone was added, and condensation was carried out at 30° C. and pH 7.5. To the resulting solution was added 31.0 g of 3-sulfatoethylsulfone-1-aminobenzene, and the reaction was completed at 70°C and pH 2.5. The reaction solution was filtered to remove insoluble matter, and then salted out using 150 g of sodium chloride. The obtained crystals were dried to obtain 92.5 g of the compound of formula 16 as shown below:
[0061]
Embodiment 2
[0063] 51.8 g of the compound of formula 15 was dissolved in 500 g of water, and 100 g of ice was added to the resulting solution, thereby cooling the solution. 19.0 g of cyanuric chloride was added to the solution, and the mixture was stirred at 5° C. and pH 5 for 2 hours to complete the reaction. Then, 22.5 g of 2-aminoethyl-2'-sulfatoethylsulfone was added, and condensation was carried out at 30° C. and pH 7.5. To the resulting solution was added 9.0 g of morpholine, and the reaction was completed at 80°C and pH 9. The reaction solution was filtered to remove insoluble matter, and then salted out using 130 g of sodium chloride. The obtained crystals were dried to obtain 81.5 g of the compound of formula 17 as shown below:
[0064]
Embodiment 3
[0066] 43.8 g of the compound of formula 18 shown below was dissolved in 500 g of water, and 100 g of ice was added to the resulting solution, thereby cooling the solution. 19.0 g of cyanuric chloride was added to the solution, and the mixture was stirred at 5° C. and pH 5 for 2 hours to complete the reaction. Then, 31.3 g of 2-(N-ethylamino)ethyl-2'-sulfatoethylsulfone was added, and condensation was performed at 25°C and pH 7.5, thereby completing the reaction. The reaction solution was filtered to remove insoluble matter, and salted out using 130 g of sodium chloride. The obtained crystals were dried to obtain 83.5 g of the compound of formula 19 as shown below:
[0067]
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com