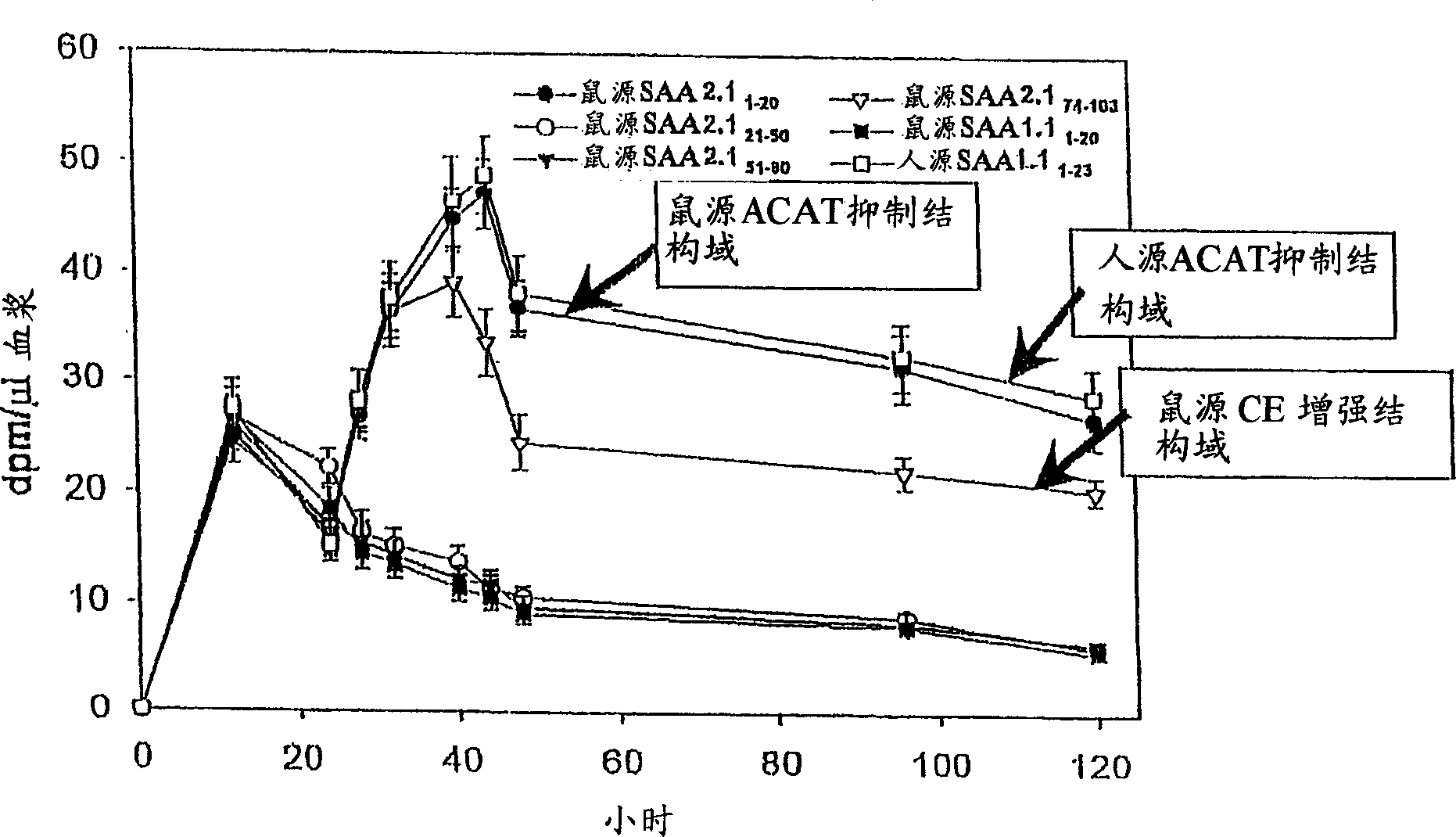
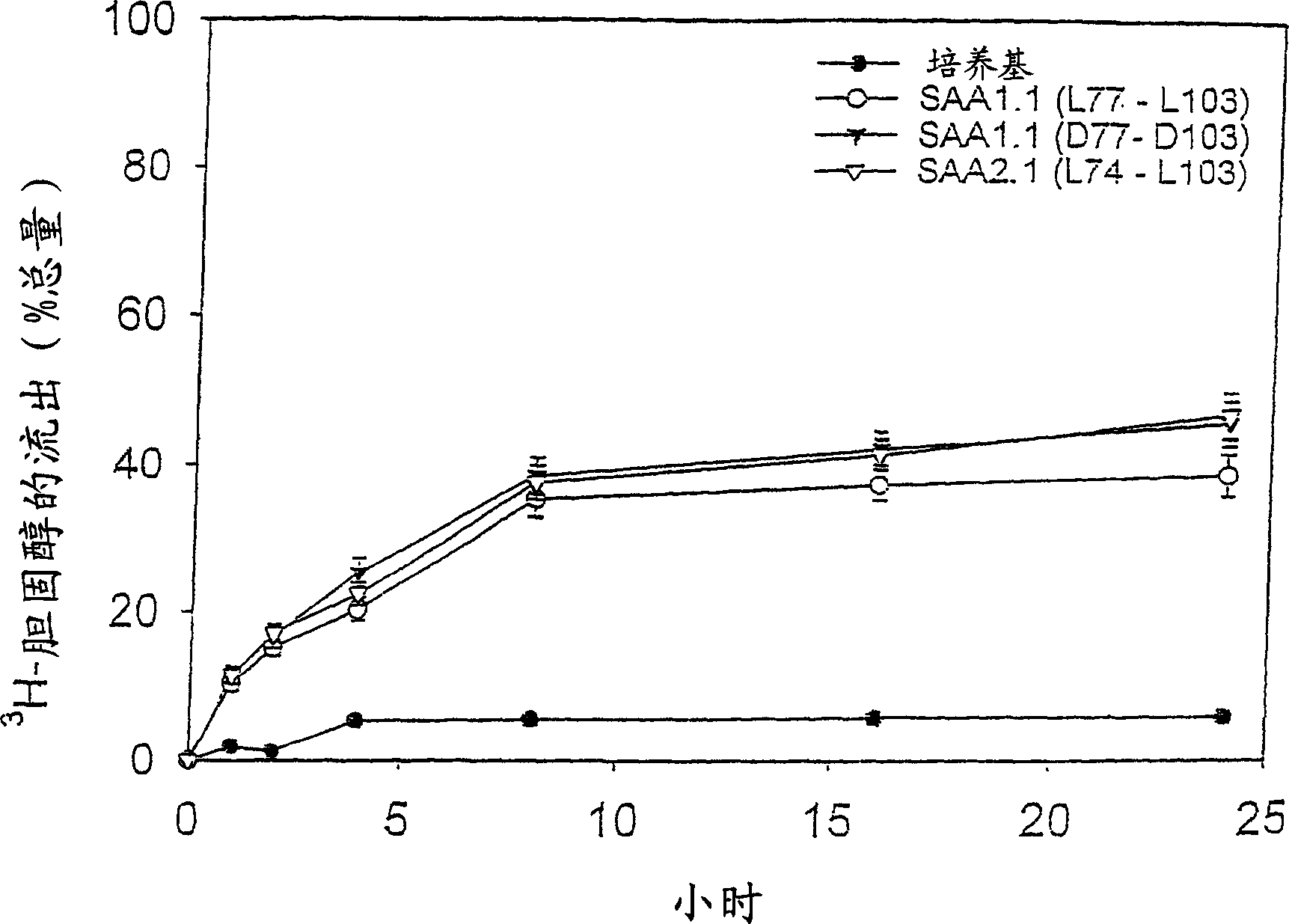
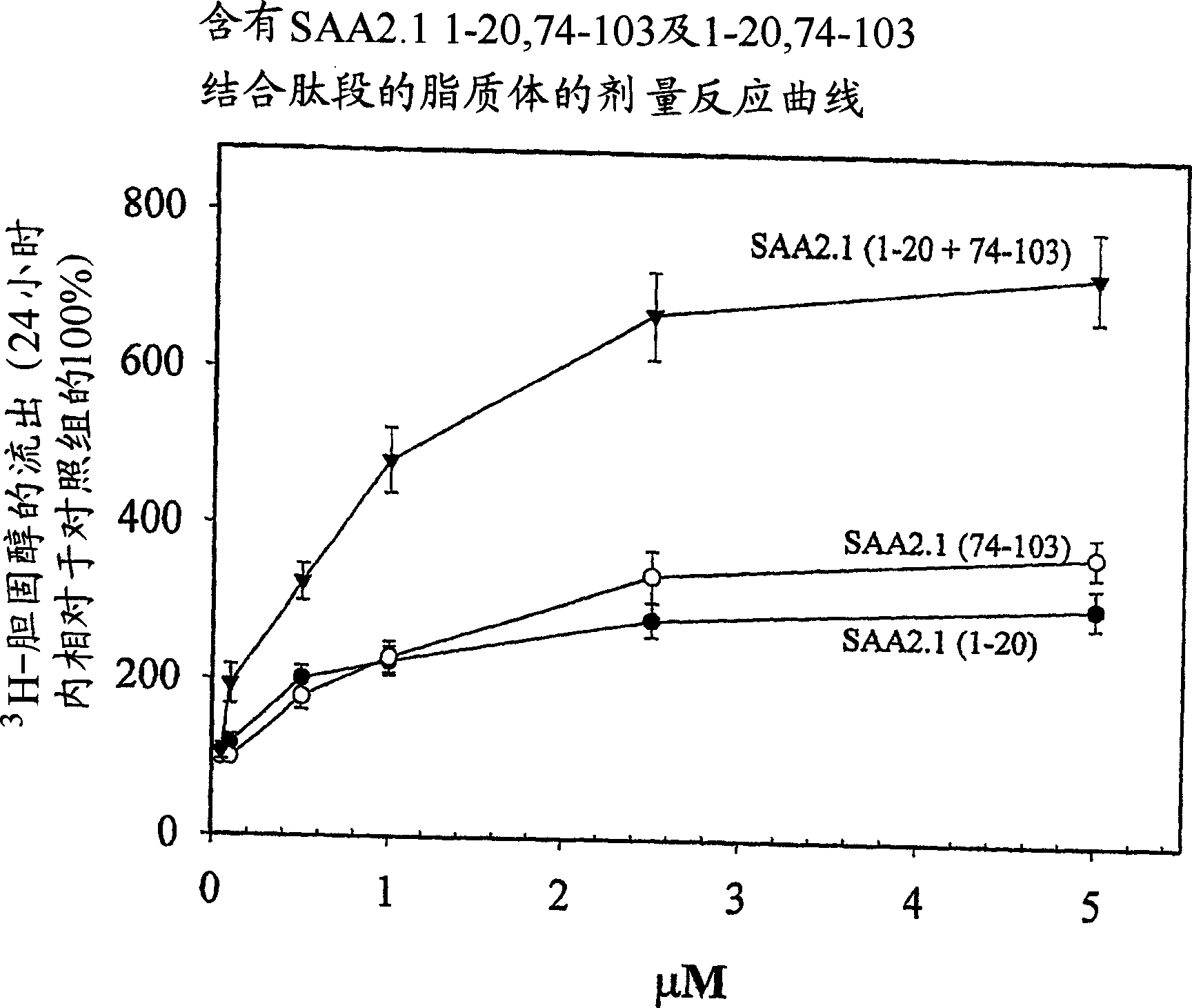
Peptides enhancing CEH activity or ACAT inhibitory activity, pharmaceutical compositions comrising these peptides and their use in the treatment of atherosclerosis
A peptide and active technology, applied in the field of activating cholesterol mobilization and promoting release, can solve problems such as accelerated arteriosclerosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0198] Example 1: Animals
[0199] 6-8 week-old female SD rats were obtained from Charles River, Montreal, Quebec, and placed in a 12-hour light-12-hour light-dark environment at constant temperature. Feed Purina laboratory mouse food tablets and water containing chlordiazepoxide.
[0200] ApoE knockout rats were obtained from Jackson Laboratory, Maine, USA.
Embodiment 2
[0201] Embodiment 2: chemical reagent
[0202] All chemicals were of reagent grade and purchased from Fisher Scientific (Nepean, Ont.), Sigma (St. Louis, MO), ICN (Aurora, OH), or BioRad (Hercules, CA). DMEM and FBS were purchased from (Burlinton, Ont.) radiolabeled [1-14C]-oleic acid (52mCi / ml), [1,2,6,7-3H(N)]-cholesterol (82mCi / ml ) was obtained from DuPont NEN (Boston, MA).
Embodiment 3
[0203] Example 3: Peptides
[0204] The following peptides were synthesized by solid-phase peptide synthesis in PEApplied Biosystems 433A peptide synthesizer using FMOC as the protecting group of amino acids.
[0205] GFFSFVHEAFQGAGDMWRAY (SEQ ID NO: 1)
[0206] TDMKEANWKNSDKYFHARGNYDAAQRGPGG (SEQ ID NO: 2)
[0207] VWAAEKISDGREAFQEFFGRGHEDTIADQE (SEQ ID NO: 3)
[0208] DTIADQEANRHGRSGKDPNYYRPPGLPDKY (SEQ ID NO: 4)
[0209] GFFSFIGEAFQGAGDMWRAY (SEQ ID NO: 5)
[0210] RSFFSFLGEAFDGARDMWRAYSD (SEQ ID NO: 6)
[0211] RGFFSFIGEAFQGAGDMWRAY (SEQ ID NO: 7)
[0212] ADQEANRHGRSGKDPNYYRPPGLPDKY (SEQ ID NO: 8)
[0213] ADQEANRHGRSGKDPNYYRPPGLPAKY (SEQ ID NO: 9)
[0214] ADQAANKWGRSGRDPNHFR (SEQ ID NO: 11)
[0215] ADQAANEWGRSGKDPNHFR (SEQ ID NO: 12)
[0216] The separation and purification of the peptides was accomplished by analytical HPLC and ion spray mass spectrometry, and the peptides were dialyzed with distilled water and freeze-dehydrated before use. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com