Lithium-ion cell composite carbon negative polar material and preparing method
A technology for lithium ion batteries and negative electrode materials, applied in battery electrodes, circuits, electrical components, etc., can solve the problems of increasing the internal resistance of the battery, affecting the cycle performance, and destroying the electrode structure, and achieves easy operation, simple preparation process, and cost. low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Example Embodiment
[0025] The preparation method of the composite carbon negative electrode material for lithium ion batteries of the present invention includes the following steps: 1. A graphite powder with a particle size of 0.3 to 30 μm and a fixed carbon content of ≥90.0%, and a binder with a weight of 1-40% of the graphite powder, Mixing and granulating, adding graphite powder and graphitization catalyst with 0.01-10% of binder weight at the same time; 2. Extruding the mixed pellets into a shape with a density of 1.3-1.9g / cm after molding 3 3. Carry out carbonization or graphitization of the molding material, heat it in a protective gas from 450°C to 3000°C, keep it for 1 to 10 hours, and then drop it to room temperature in a protective atmosphere; 4. Crush the above-mentioned composite carbon material; 3. Crush the above-mentioned composite carbon material; 5. Carry out purification treatment.
[0026] The graphite micropowder in the preparation method of the lithium ion battery composite carb...
Example Embodiment
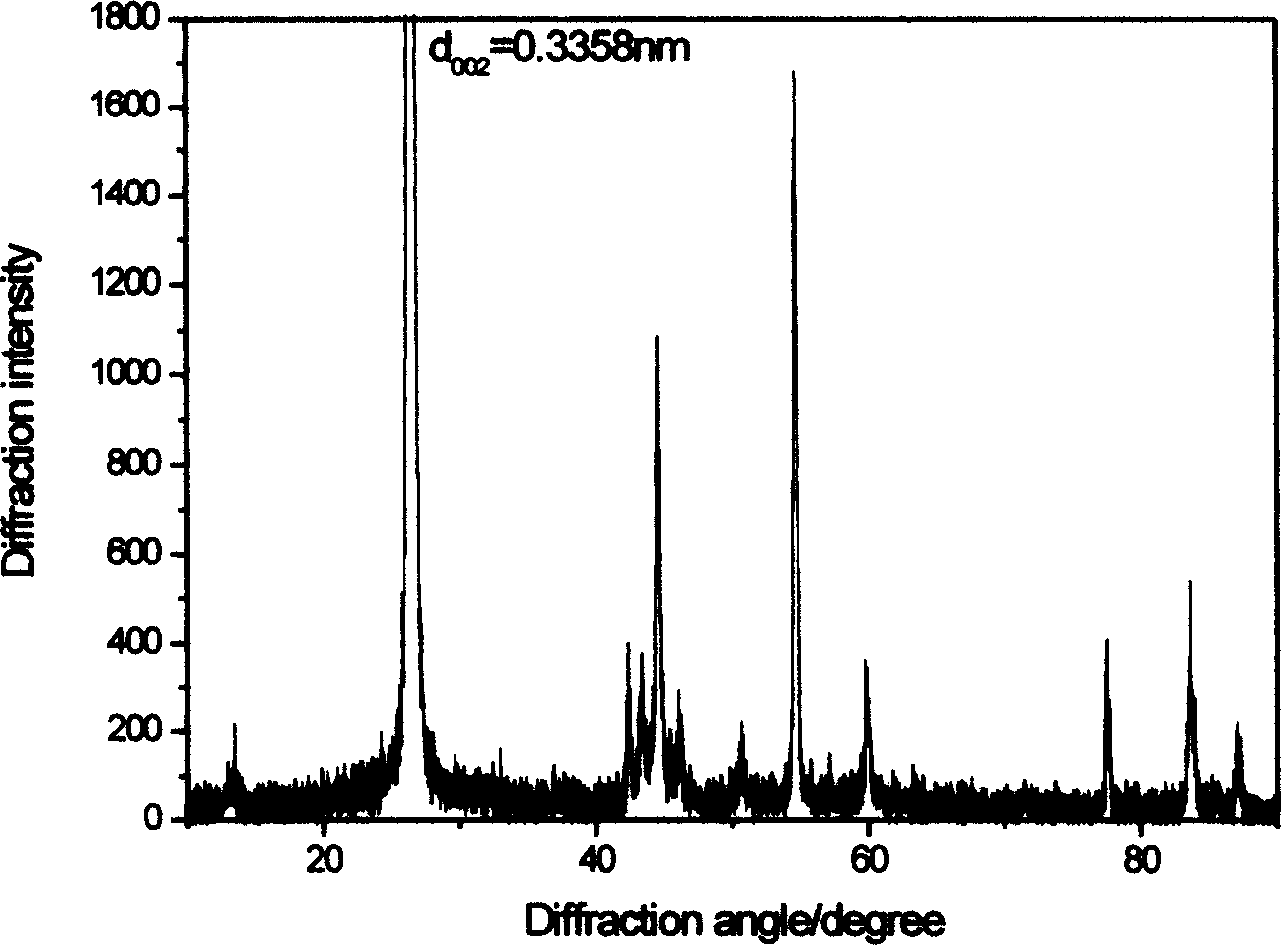
[0046] In Example 1, 100 parts by weight of natural graphite powder with an average particle size of 5μm and a carbon content of 93.6% were added to 20 parts by weight of petroleum pitch, 10 parts by weight of coal tar, and 10 parts of silicon carbide (the amount of addition of Si equivalent to 5.3wt%) The parts by weight are mixed and granulated at a temperature of 150°C and pressed into cylindrical graphite blocks with a pressing density of 1.9g / cm 3 , Graphitized at a temperature of 2800°C for 4 hours, then cooled to room temperature, and pulverized with a high-pressure mill to obtain composite carbon particles with an average particle size of 20μm. Such as figure 2 As shown, it can be seen from the scanning electron microscope photo of the composite carbon particles that the composite carbon particles are secondary particles formed by combining multiple graphite powders. This is measured by the JEOLJSM-6380LV scanning electron microscope and the Millitrac particle image analy...
Example Embodiment
[0048] In Example 2, 50 parts by weight of natural graphite powder with an average particle size of 10 μm and a carbon content of 96.4% and 50 parts by weight of artificial graphite powder with the same particle size and carbon content were mixed uniformly, 10 parts by weight of petroleum pitch and 20 parts by weight of coal tar were added. , 17 parts by weight of boron carbide (10wt% equivalent to B) is mixed and granulated at a temperature of 150°C, and compressed into cylindrical graphite blocks with a density of 1.65g / cm 3 Graphitized at 3000°C for 1 hour, then cooled to room temperature, and pulverized with a low-speed impact spheroidizing mill to obtain composite carbon particles with an average particle size of 20 μm. From the scanning electron micrograph of the composite carbon particles, it can be seen that the composite carbon particles are secondary particles combined with a number of graphite micropowders. The length-to-diameter ratio of the composite carbon material i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average particle size | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
| Crystal size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap