Method for predicting angiotonin II receptor agonist hypotensor function and use
A technology for angiotensin and antagonists, applied in the field of predicting the action of angiotensin II receptor antagonist antihypertensive drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
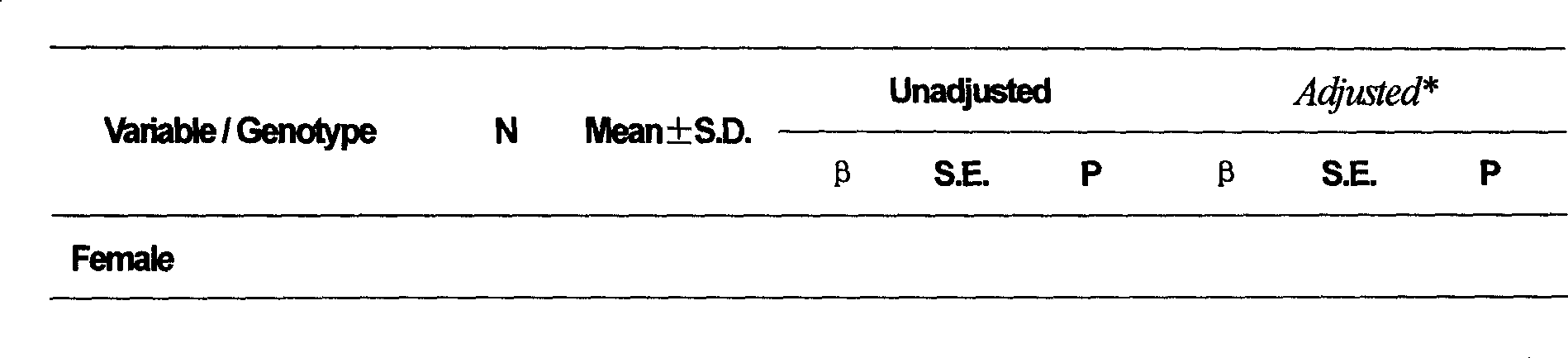
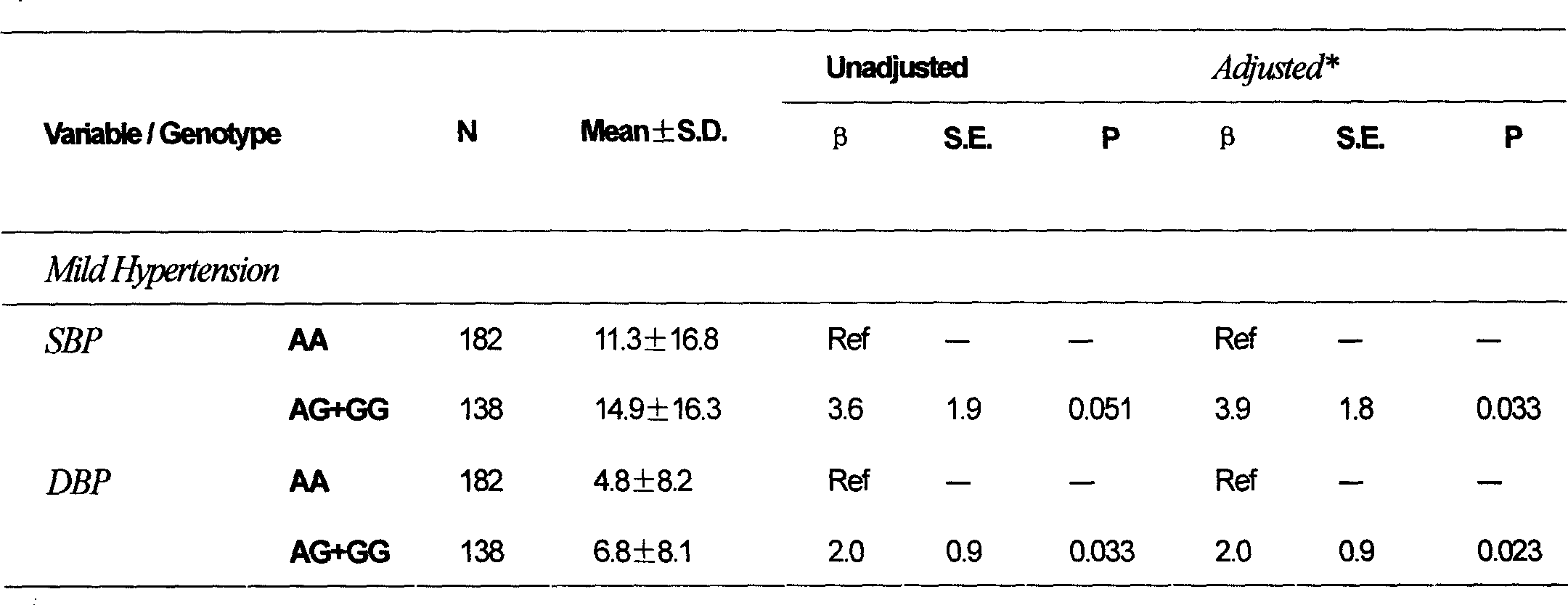
[0081] Example 1: Determination of the Ala222Val polymorphism site of the MTHFR gene and prediction of the antihypertensive effect of the AT1 receptor antagonist antihypertensive drug nifedipine
[0082] (1) Determination of the genotype of the Ala222Val polymorphism site of the MTHFR gene:
[0083] (1) Extract the genomic DNA of the host cell:
[0084] (a) Add 30ml of erythrocyte lysate to the whole blood, shake slowly, and let stand at room temperature for 10 minutes. During this period, shake several times to completely lyse the erythrocytes;
[0085] (b) Centrifuge at 4°C at 2000 rpm for 10 minutes, remove the supernatant, break up the precipitated leukocytes on a rotary shaker, add 40ul of protease and 50ul of RNase, shake well, add 15ml of leukocyte lysate, Mix well in a 37°C water bath for 20 minutes, take it out, and put it in cold water;
[0086] (c) Add 4ml of cold protein precipitation solution, mix well and place in -20°C refrigerator for 5 minutes, take it out a...
Embodiment 2
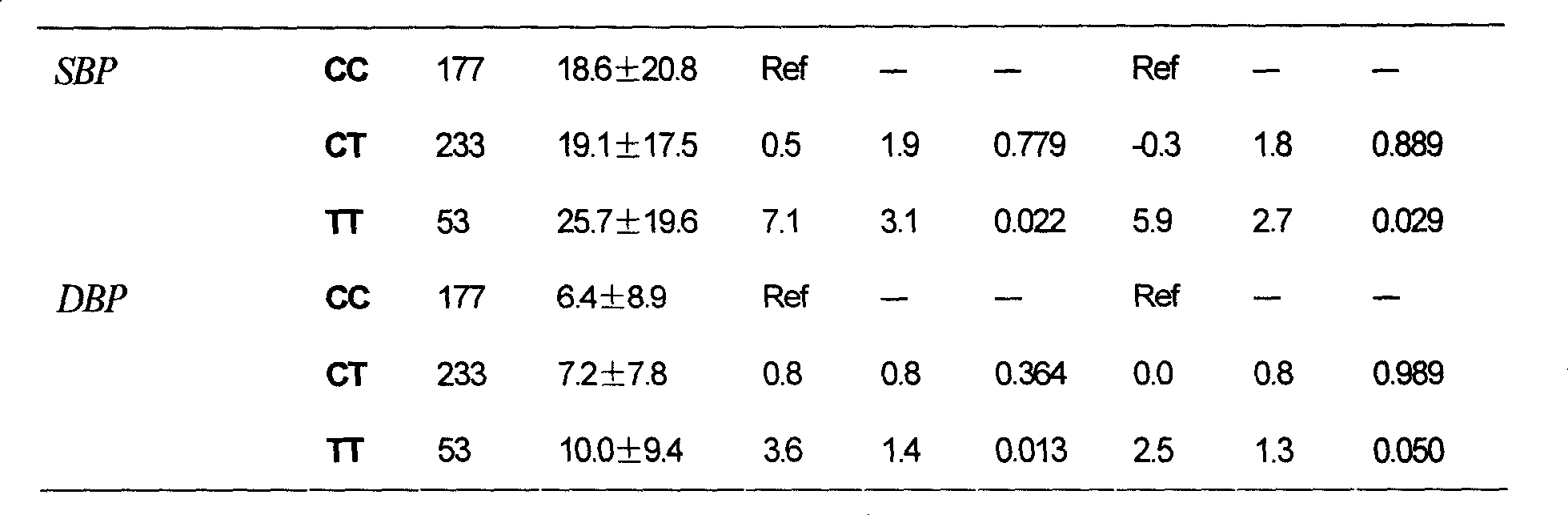
[0118] Example 2: Determining the genotype of the MTRR gene Ile22Met (A145G) polymorphism and predicting the antihypertensive effect of the AT1 receptor antagonist antihypertensive drug nifedipine
[0119] (1) DNA extraction is the same as in Example 1, and the polymorphic site genotype determination method is similar to Example 1, wherein
[0120] The polymorphic site of the MTHFR Ile22Met gene and its flanking sequences were amplified with a PCR instrument. Corresponding to the "A (or Ile)" allele, the specific probe carries the VIC fluorescent reporter group; corresponding to the "G (or Met)" allele, the specific probe carries the FAM fluorescent reporter group.
[0121] Put the PCR plate that completed the PCR reaction into the 7900 fluorescent quantitative PCR instrument, and select the "AllelicDiscrimination" program to scan and judge the results:
[0122] The genotype of those who emit FAM fluorescence is Met / Met homozygote;
[0123] The genotype of those who emit VIC...
Embodiment 3
[0133] Embodiment 3: Measuring the Ala222Val polymorphic site kit composition (PCR-RFLP method) and application of MTHFR gene
[0134] 1. Kit components include:
[0135] Solution A: PCR buffer (PCR buffer), the components are KCL, Tris-HCl and MgCl2;
[0136] Solution B: deoxymononucleotide triphosphate (dNTP);
[0137] Liquid C: primer (Primer), synthesized by an oligonucleotide synthesizer; the sequence of the primer in the kit is:
[0138] Upstream primer: 5'-CAAAGGCCACCCCGAAGC-3', sequence listing 3 (SEQ ID No.3)
[0139] Downstream primer: 5'-AGGACGGTGCGGTGAGAGTG-3', sequence listing 4 (SEQ ID No.4)
[0140] Solution D: heat-resistant DNA polymerase (Taq); storage temperature is -20°C;
[0141] Solution E: restriction endonuclease HinfI; storage temperature is -20°C;
[0142] Solution F: restriction endonuclease buffer, the components are Tris-HCl, NaCl, MgCl2, dithiothreitol.
[0143] 2. The steps of using this kit to amplify functional polymorphic sites and their...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com