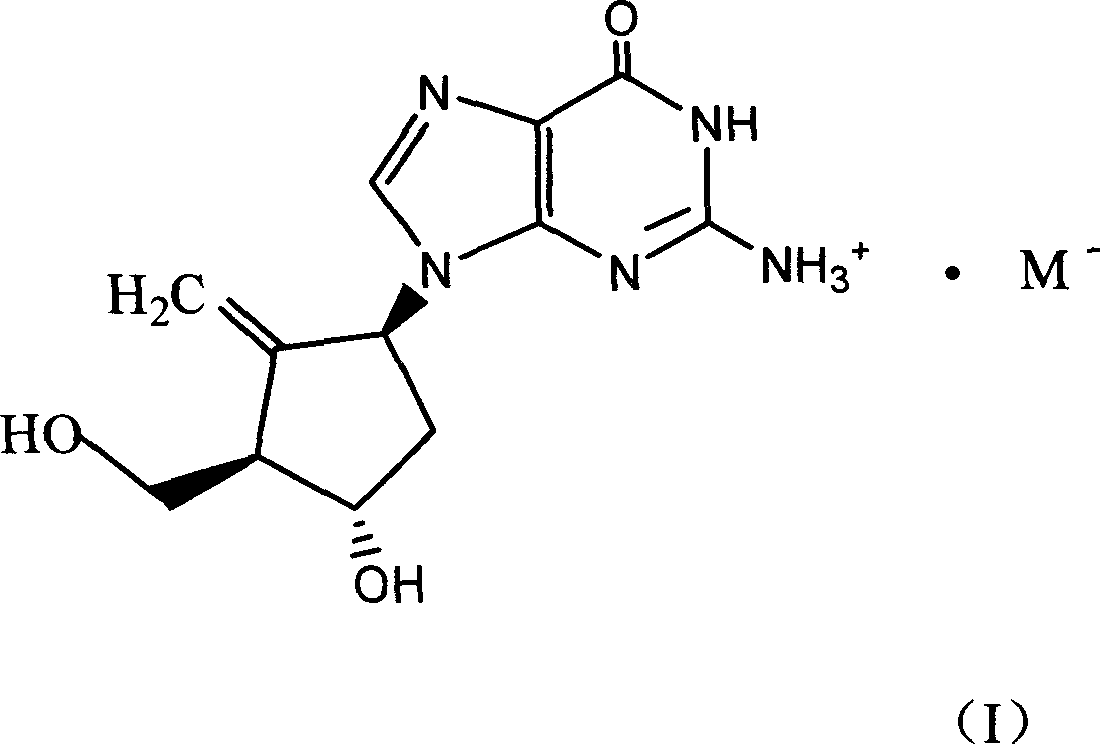
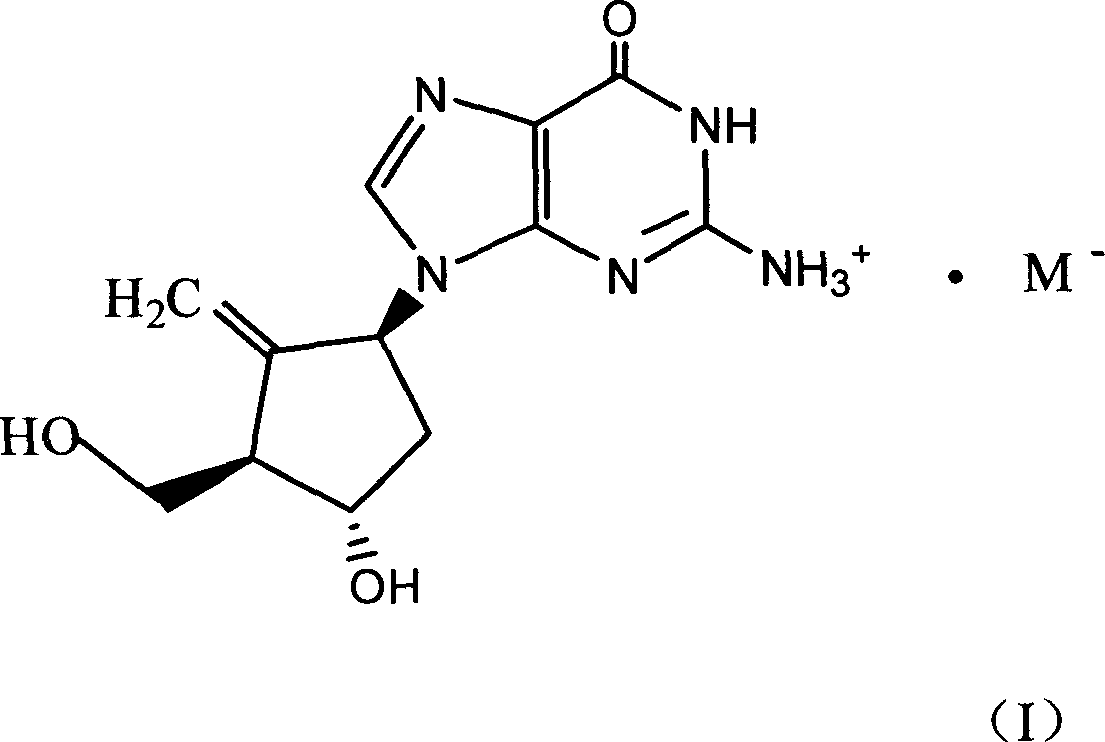
Entecavir acid addition salt, preparation method and use thereof
A technology of endecavir and acid addition salt, which is applied in the field of endecavir acid addition salt and its preparation and use, and can solve the problems of low solubility of endecavir and unfavorable preparation of pharmaceutical preparations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 [1S-(1α, 3α, 4β)]-2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl] -6H-purin-6-one hydrochloride
[0025] [1S-(1α,3α,4β)]-2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H - Purin-6-one (2.8g) and hydrochloric acid (2ml of 6N hydrochloric acid) were dissolved in 500ml of absolute ethanol, heated to reflux until dissolved, the hot solution was filtered through celite, then slowly cooled under gentle stirring, placed in 0- It was placed in a refrigerator at 5°C for several hours, and the endecavir hydrochloride was filtered out, washed with absolute ethanol, and dried under vacuum at 40°C to obtain 2.2 g of product with a yield of 70%, m.p. 240-243°C.
Embodiment 2
[0026] Example 2 [1S-(1α, 3α, 4β)]-2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl] -6H-purin-6-one maleate
[0027] [1S-(1α,3α,4β)]-2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]-6H -Purin-6-one (2.8g) and maleic acid (1.2g) were dissolved in 800ml of absolute ethanol, heated to reflux until dissolved, the hot solution was filtered through celite, then slowly cooled under gentle stirring, at 0 After standing in a -5°C refrigerator for several hours, the endecavir maleate was filtered out, washed with absolute ethanol, and vacuum-dried at 40°C to obtain 2.75 g of product, yield 70%, m.p. 210-212°C.
Embodiment 3
[0028] Example 3 Accelerated Stability Test
[0029] Sample
Taste
time
(moon)
Exterior
Content (%)
A
0
1
2
3
6
off-white
off-white
off-white
off-white
off-white
98.79
98.69
98.61
98.55
98.50
B
0
1
2
3
6
off-white
off-white
off-white
off-white
off-white
99.21
99.06
99.03
99.00
98.88
C
0
1
2
3
6
off-white
off-white
off-white
off-white
off-white
99.51
99.36
99.20
99.05
98.86
[0030] A: [1S-(1α,3α,4β)]-2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]- 6H-purin-6-one hydrochloride
[0031] B: [1S-(1α,3α,4β)]-2-amino-1,9-dihydro-9-[4-hydroxy-3-(hydroxymethyl)-2-methylenecyclopentyl]- 6H-purin-6-one maleate
[0032] C: [1S-(1α,3α,4β)]-2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com