Novel method for the preparation of intermediates useful for the synthesis of vitamin d analogues
A compound and halogen technology, applied in the field of manufacturing calcipotriol or calcipotriol monohydrate, can solve the problems of difficult removal, increased chromatography steps, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
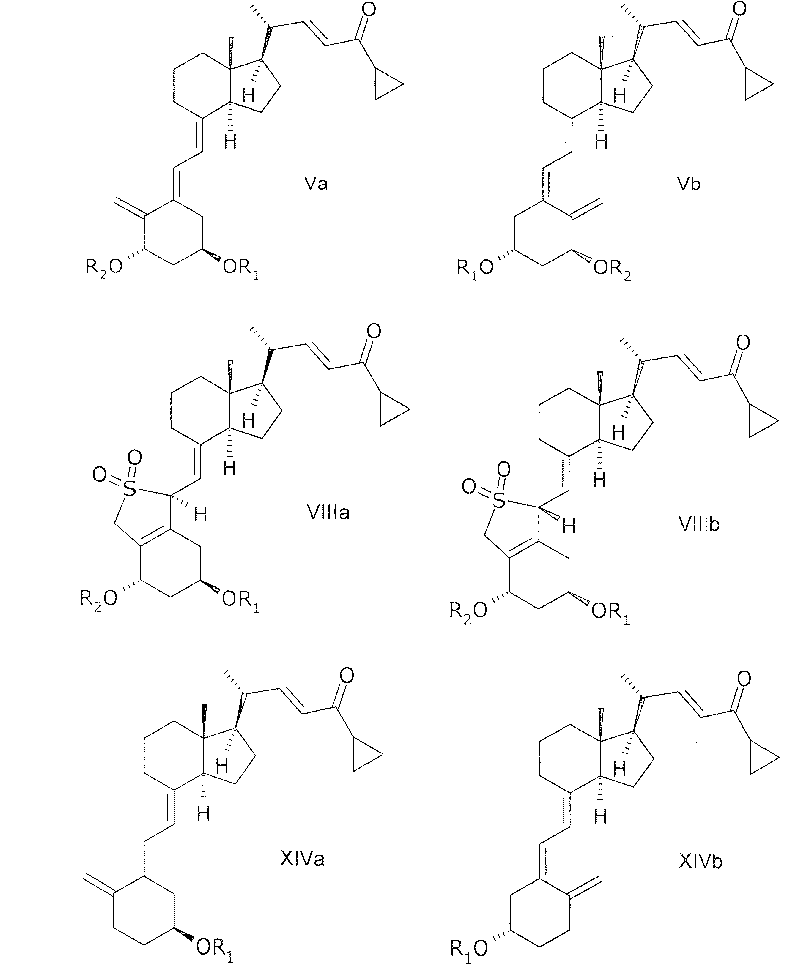
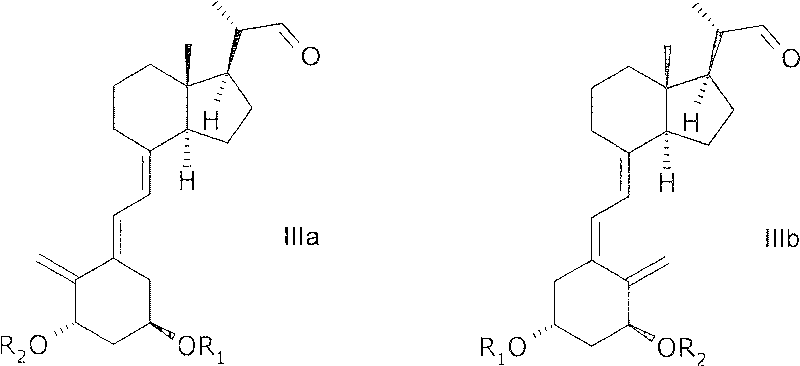
[0174] In another aspect, the invention relates to 20(R), 1(S), 3(R)-bis(tertiary) obtained by a process comprising reacting a compound of general structure IIIa with a phosphonate of general structure VII. Butyldimethylsilyloxy)-20-(3'-cyclopropyl-3'-oxoprop-1'(E)-enyl)-9,10-depregna-5(E) , 7(E), 10(19)-triene.
[0175] In another aspect, the invention relates to 20(R), 1(S), 3(R)-bis(tertiary) obtained by a process comprising reacting a compound of general structure IIIb with a phosphonate of general structure VII. Butyldimethylsilyloxy)-20-(3'-cyclopropyl-3'-oxoprop-1'(E)-enyl)-9,10-depregna-5(Z) , 7(E), 10(19)-triene.
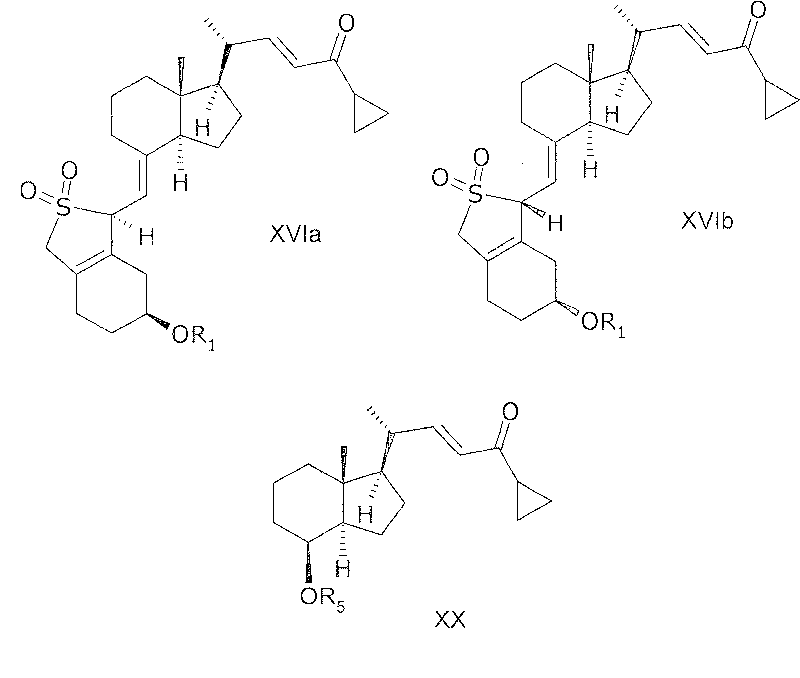
[0176] In another aspect, the invention relates to 20(R), 1(S), 3(R)-bis(R), obtained by a process comprising reacting a compound of general structure VIa or VIb with a phosphonate of general structure VII. (tert-butyldimethylsilyloxy)-20-(3'-cyclopropyl-3'-oxoprop-1'(E)-enyl)-9,10-depregna-5( E), SO of 7(E), 10(19)-triene 2 adduct.
[0177] In a pres...
Embodiment 1
[0231] 20(R), 1(S), 3(R)-bis(tert-butyldimethylsilyloxy)-20-(3'-cyclopropyl-3'-oxopropane-1'(E )-alkenyl)-9,10-depregna-5(E),7(E),10(19)-triene
[0232] Compound Va(R 1 , R 2 = tert-butyldimethylsilyl)
[0233] Diethyl (2-cyclopropyl-2-oxoethyl)phosphonate (compound VII / R 3 , R 4 = ethyl) (46.0 g, 209 mmol), 1(S), 3(R)-bis(tert-butyldimethyl) prepared according to M.J. Calverley, Tetrahedron, Vol. 43, p. 20, pp. 4609-4619 1987 Methylsilyloxy)-20(S)-formyl-9,10-depregna-5(E),7(E),10(19)-triene (compound IIIa / R 1 , R 2 = tert-butyldimethylsilyl) (72.2g, 126mmol), toluene (1100ml), water (122ml), tetrabutylammonium bromide (3.13g) and sodium hydroxide solution 27.7% (128.0g) The mixture was stirred at 30°C for about one hour, then at room temperature (15-25°C) overnight. When the reaction was complete as judged by HPLC [ColumnLiChrosorb Si 60 5 μm 250 x 4 mm from Merck, flow rate 1.5 ml / min, detection at 270 nm, hexane / ethyl acetate 100:2 (v:v)], water was added ( 500ml)...
Embodiment 1A
[0235]20(R), 1(S), 3(R)-bis(tert-butyldimethylsilyloxy)-20-(3'-cyclopropyl-3'-oxopropane-1'(E )-alkenyl)-9,10-depregna-5(E),7(E),10(19)-triene
[0236] Compound Va(R 1 , R 2 = tert-butyldimethylsilyl)
[0237] To (2-cyclopropyl-2-oxoethyl) phosphonic acid diethyl ester (compound VII / R 3 , R 4 =ethyl) (1.51 g) in THF (16 ml) was added NaHMDS (sodium hexamethyldisilazane) (3.2 ml, 2M in THF) over 10 minutes below -50 °C, and Stir for another 3-4 hours, then add 1(S),3(R)-bis(tert-butyldimethylsilyloxy)-20(S)-formyl-9 below -50 °C, 10-pregna-5(E), 7(E), 10(19)-triene (compound IIIa / R 1 , R 2 = tert-butyldimethylsilyl) (2 g) in THF (3 ml). The reaction was stirred at below -50°C for an additional 2 hours before the temperature was allowed to warm to room temperature overnight, and then at -25°C for 2 hours. The reaction was checked for completion by HPLC [ColumnLiChrosorb Si 60 5 μm 250×4 mm from Merck, flow rate 1.5 ml / min, detection at 270 nm, hexane / ethyl acetate 100:2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com