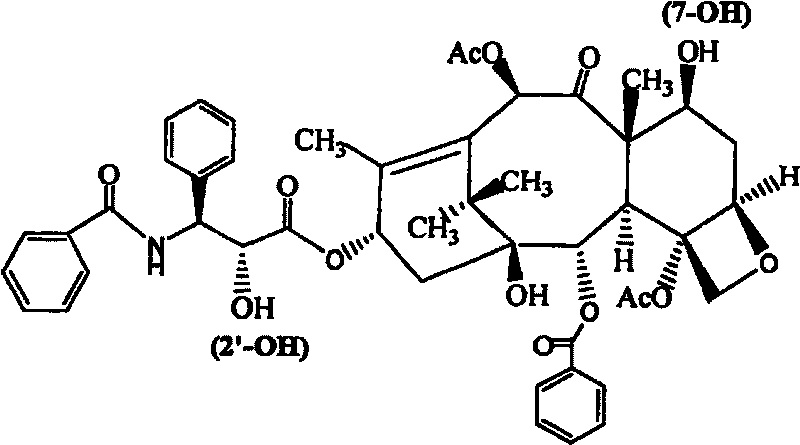
Amphiphilic tri-block copolymer taxol bonding medicament and synthesis method thereof
A copolymer and tri-block technology, which is applied in the field of paclitaxel-bonded drugs and its synthesis, can solve the problems of adverse effects of preparation manufacturing, difficulties in the synthesis of carrier polymers, difficulty in large-scale production and clinical application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1: the preparation of PLA-PEG-PLA triblock polymer
[0036] 1 g of lactide (LA) monomer recrystallized three times with ethyl acetate and 4 g of polyethylene glycol (PEG) with a molecular weight of 4600 were added to a water separator with a reflux condensing unit that was ventilated three times with high-purity argon. tube and a dry ampoule with a magnetic stirrer, add anhydrous toluene solvent with a total mass ratio of LA and PEG of 2:1 to azeotropically remove water, then distill off half of the toluene, add about 0.2ml molar concentration of 2× 10 -3 mol / l stannous octoate toluene solution. Stir and react at 110°C for 12h, then dissolve the product in an appropriate amount of dichloromethane, settle with ether to obtain a white product, dry it in vacuum at 40°C to obtain a PLA-PEG-PLA triblock polymer, and calculate by NMR The total molecular weight of the two PLA blocks is about 1100.
Embodiment 2
[0037] Embodiment 2: the preparation (solution method) of the PLA-PEG-PLA triblock polymer of terminal carboxyl group
[0038] Dissolve 1.0g of hydroxyl-terminated PLA-PEG-PLA triblock polymer in 20ml of 1,4-dioxane, and then add 0.024g of succinic anhydride, 0.029g of DMAP and 0.03ml of TEA at 0°C. Stir the reaction for 24 hours. Filter out the resulting precipitate, concentrate the filtrate and settle it with a large amount of ether, filter, and vacuum-dry at 40°C to obtain a white product of PLA-PEG-PLA triblock polymer with carboxyl groups.
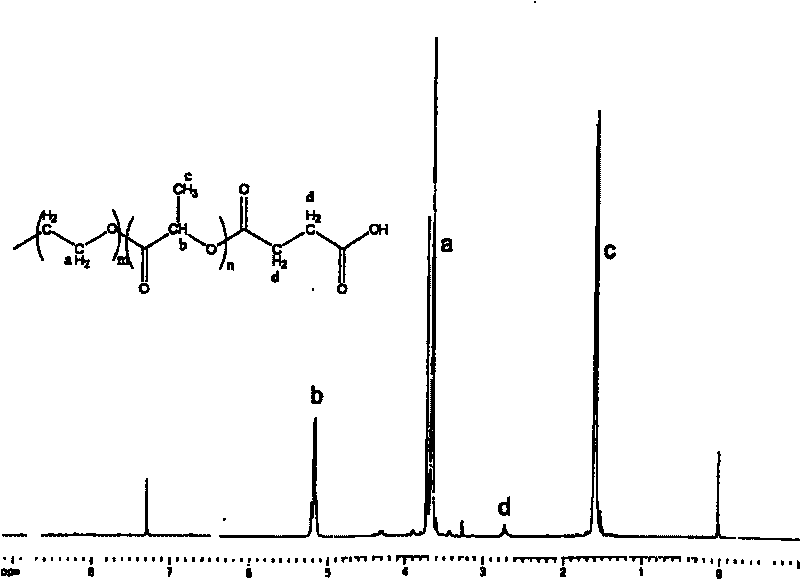
[0039] Gained end group is the nuclear magnetic spectrum of the PLA-PEG-PLA triblock polymer of carboxyl group see image 3 .
Embodiment 3
[0040] Embodiment 3: the preparation (melt method) of the PLA-PEG-PLA triblock polymer of terminal carboxyl group
[0041] Put 0.5g of hydroxyl-terminated PLA-PEG-PLA triblock polymer and 0.012g of succinic anhydride into a one-necked bottle, heat to 130°C until the polymer melts, maintain a constant temperature, react for 8 hours, add 10ml of chloroform to dissolve, Settled with a large amount of ether, filtered, and dried under vacuum at 40°C to obtain a white product of carboxyl-terminated PLA-PEG-PLA triblock polymer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com