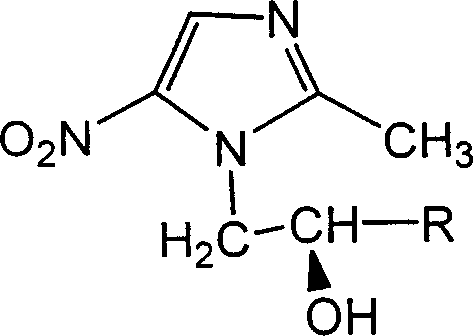
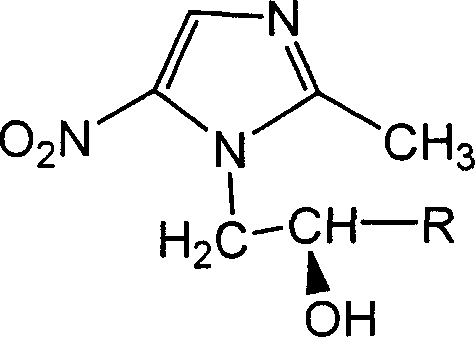
Alpha-substituted-2-methyl-5-nitro-diazole-1-alcohol derivative with optical activity
A technology of nitroimidazole and derivatives, which is applied in the field of preparation of anti-anaerobic bacteria drugs, can solve the problems that the optical activity of 2-methyl-5-nitroimidazole-1-ethanol derivatives has not been reported, and achieve good anti-anaerobic bacteria activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
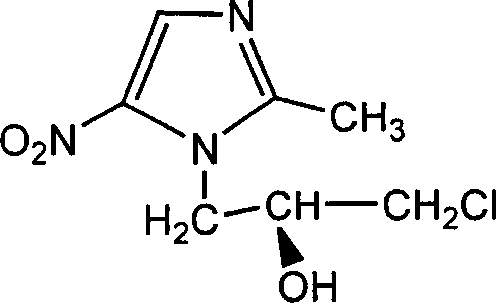
[0037] Preparation of S-(-)-1-(2,3-epoxypropyl)-2-methyl-5-nitroimidazole
[0038] Add 100g (0.45mol) S-(-)-1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole and 200ml ethanol into the reaction flask, and cool to 5~ At 10°C, 270ml (0.68mol) of 10% NaOH aqueous solution was added dropwise, and the temperature was kept below 20°C for 30 minutes after the drop was completed. After the reaction, the reaction solution was poured into 200 ml of water, extracted with ethyl acetate, and the organic layers were combined and dried over anhydrous magnesium sulfate. Filter and evaporate to dryness under reduced pressure to obtain 81.5 g of oily substance. [α] D 20 =-27.7° (C=1.0, ethanol)
Embodiment 2
[0040] Preparation of R-(-)-1-(2-methyl-5-nitroimidazol-1-yl)-3-morpholinopropan-2-ol
[0041] Heat 10g (54.6mmol) S-(-)-1-(2,3-epoxypropyl)-2-methyl-5-nitroimidazole, 80ml methanol, and 4.7ml (54mmol) morpholine under reflux for 3h , slightly cooled, evaporate methanol under reduced pressure, add 80ml of water to the residue, extract with ethyl acetate, combine organic layers, and dry over anhydrous magnesium sulfate. Filter, and distill off ethyl acetate under reduced pressure to obtain an oil. Dissolve the oil in 200ml of ethanol and pass through dry HCl gas to precipitate a solid, which is filtered and dried to obtain the hydrochloride. The hydrochloride was dissolved in 200ml of water, adjusted to PH=8 with 10% NaOH, extracted with ethyl acetate, and the combined organic layers were dried over anhydrous magnesium sulfate. Filter and evaporate to dryness under reduced pressure to obtain an oil. Place to crystallize to obtain 8.5 g of solid. Yield: 57.8%, mp: 82-84°C, [...
Embodiment 3
[0048] Preparation of R-(+)-1-(2,3-epoxypropyl)-2-methyl-5-nitroimidazole
[0049] Add 100g (0.45mol) R-(+)-1-(3-chloro-2-hydroxypropyl)-2-methyl-5-nitroimidazole and 200ml ethanol into the reaction flask, and cool to 5~ At 10°C, 270ml (0.68mol) of 10% NaOH aqueous solution was added dropwise, and the temperature was kept below 20°C for 30 minutes after the drop was completed. After the reaction, the reaction solution was poured into 200 ml of water, extracted with ethyl acetate, and the organic layers were combined and dried over anhydrous magnesium sulfate. Filter and evaporate to dryness under reduced pressure to obtain 81.5 g of oily substance. [α] D 20 =+28.6° (C=1.0, ethanol)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com