Derivatives of sulindac can protect normal cells against oxidative damage
a sulindac and normal cell technology, applied in the direction of antinoxious agents, drug compositions, cardiovascular disorders, etc., can solve the problems of ros can cause oxidative damage to cells and tissues, limited long-term use of non-steroidal anti-inflammatory drugs such as sulindac, and significant toxicity of sulindac sulfone relative to sulindac, etc., to achieve no cox inhibitory activity, increase intracellular cgmp and/or camp
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
working examples
[0133]The present invention is now described in terms of the following non-limiting examples.
example 1
Materials and Methods
[0134]Chemical synthesis of Compound 9: 3,4-Dimethoxybenzaldehyde was treated with propionic anhydride and sodium propionate at 130° C. for 36 h to give (Z)-3-(3,4-dimethoxyphenyl)-2-methylacrylic acid as a colorless solid. Catalytic hydrogenation at 40 psi afforded 3-(3,4-dimethoxyphenyl)-2-methylpropanoic acid as colorless oil. 5,6-Dimethoxy-2-methyl-2,3-dihydro-1H-inden-1-one was obtained by treating the oil with polyphosphoric acid (PPA) at 50° C. for 20 min, followed by a standard purification procedure; the ketone was refluxed with 2-cyanoacetic acid, acetic acid and ammonium chloride in toluene for 36h, followed by hydrolysis with potassium hydroxide in 60% of ethanol to give 2-(5,6-dimethoxy-2-methyl-1H-inden-3-yl)acetic acid. Reaction of the acid with 3,4,5-trimethoxybenzaldehyde and methoxide in methanol at 85° C. overnight afforded 2-(5,6-dimethoxy-2-methyl-1-(3,4,5-trimethoxybenzyl)-1H-inden-3-yl)acetic acid (Compound 9) as a yellow solid.
[0135]Chemi...
example 2
Identification and Initial Evaluation of Indene Derivatives
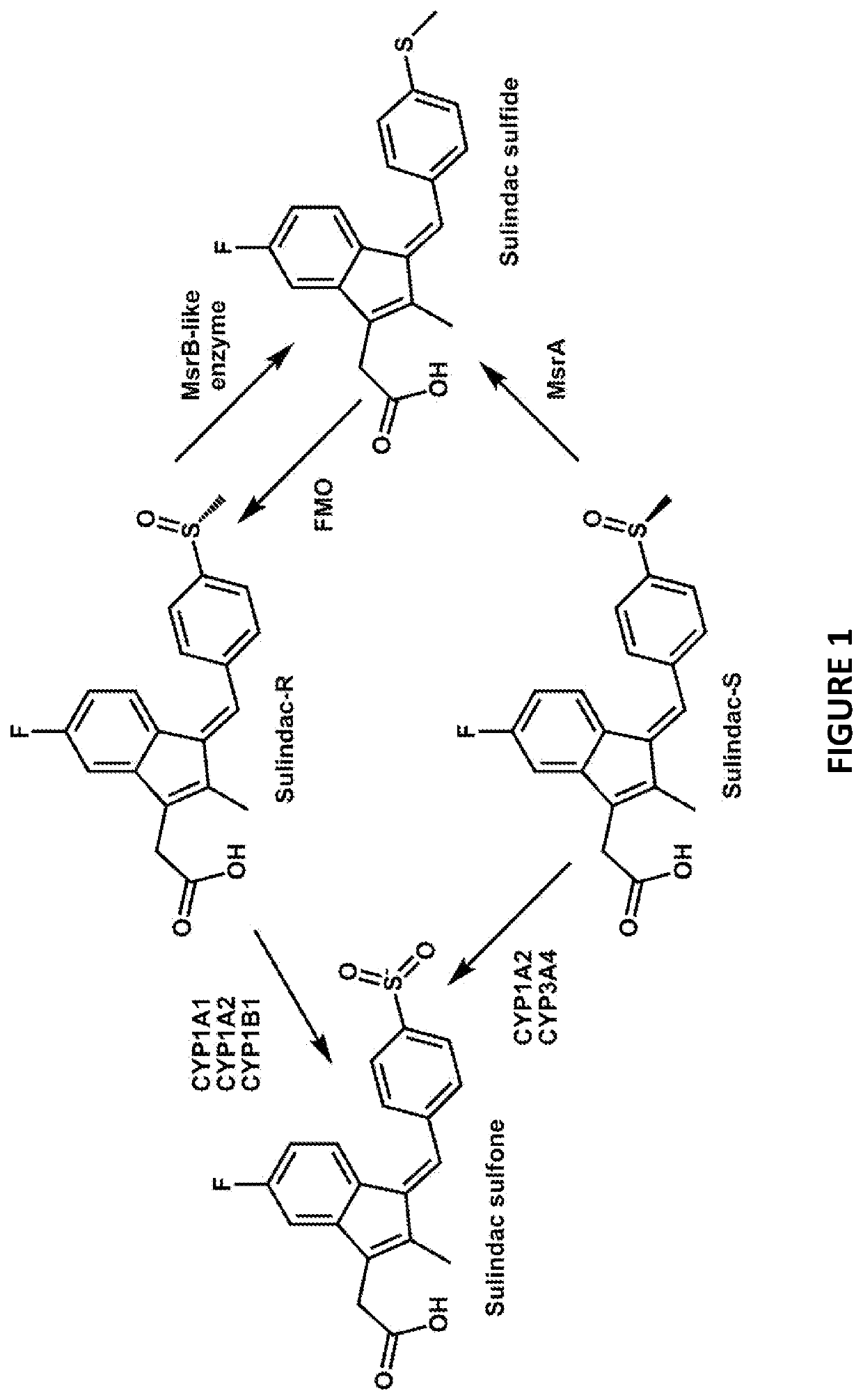
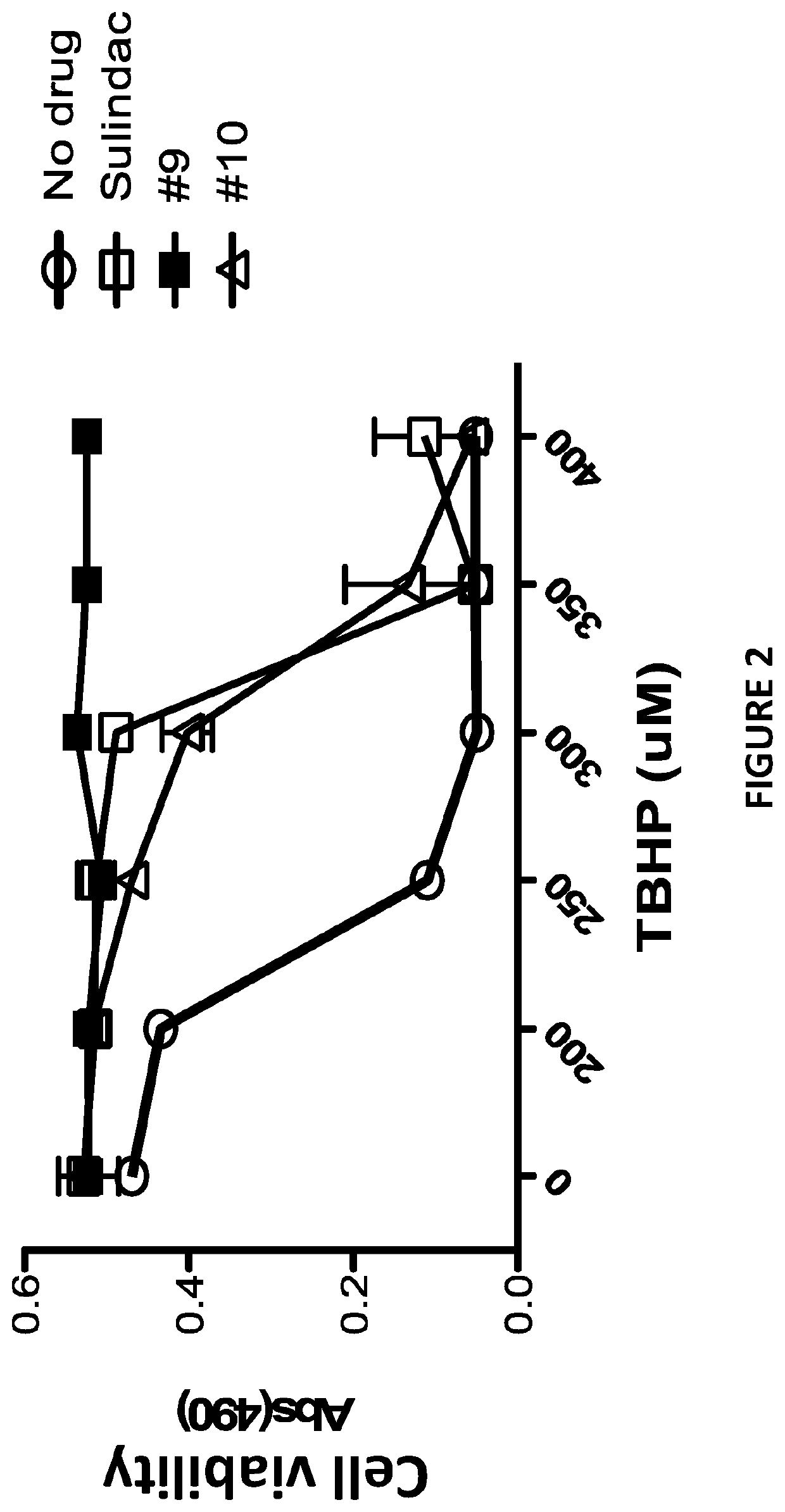
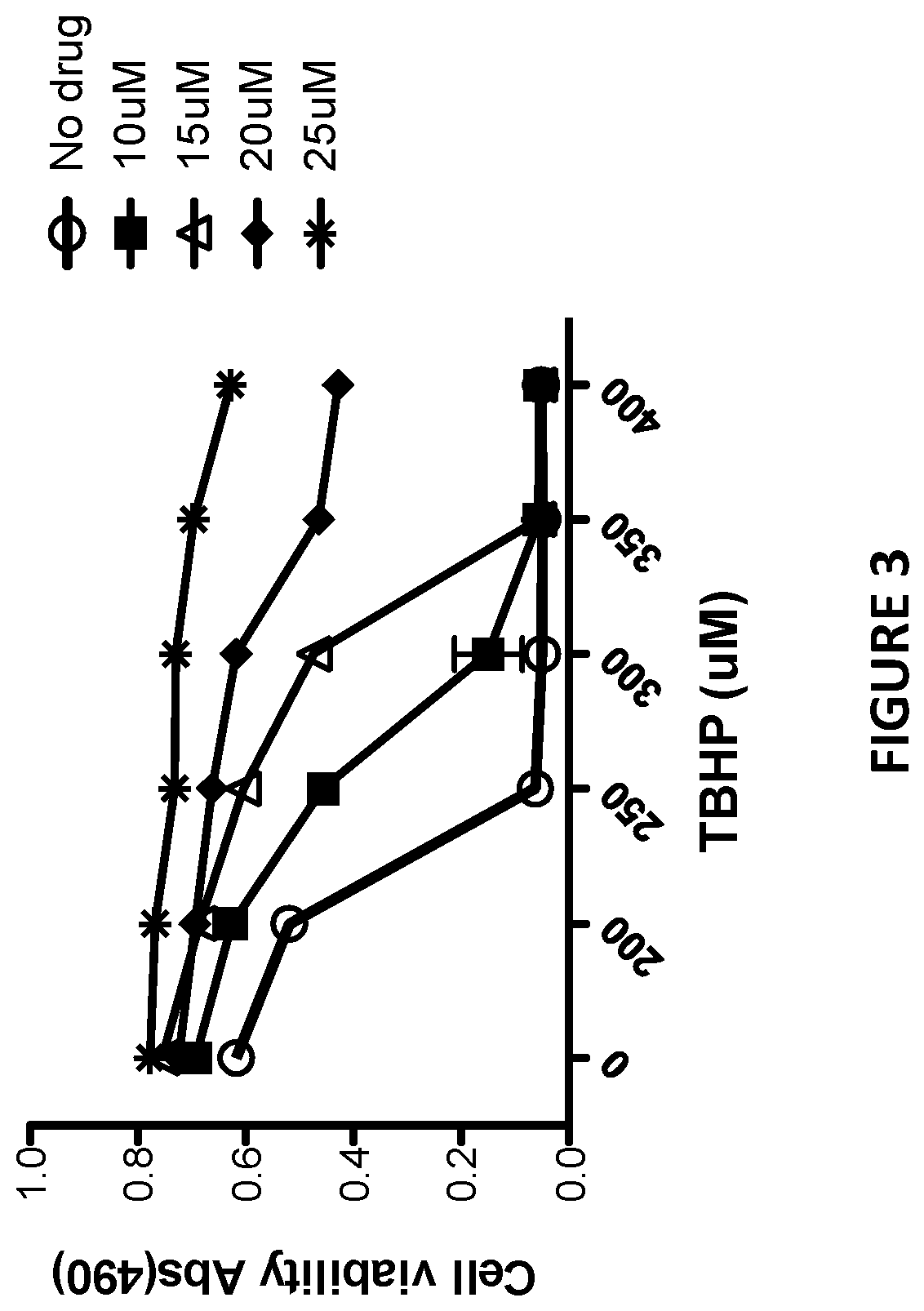
[0144]Sulindac is a known NSAID that has been shown to have anti-cancer activity in experimental rodent models of tumorigenesis and is effective for the treatment of precancerous colonic adenomas in patients with familial adenomatous polyposis (15-17). However, sulindac, like other NSAIDs, has gastrointestinal and other toxicities resulting from COX inhibition that may limit their long-term use for cancer chemoprevention or other diseases. To develop safer and more efficacious derivatives, previous studies have focused primarily on synthesizing derivatives that have reduced COX inhibitory activity and improve potency to inhibit tumor cell growth (18, 19). As shown previously, sulindac can also protect normal cells against oxidative damage by initiating a protective preconditioning response (9, 10) that is independent of its COX inhibitory activity. Since the protective effect observed with sulindac using cells in culture req...
PUM
| Property | Measurement | Unit |
|---|---|---|
| emission wavelength | aaaaa | aaaaa |
| excitation wavelength | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com