Nucleotide sequence encoding a modulator of NF-kappaB
a technology of nf-kappab and nucleotide sequence, which is applied in the direction of peptide/protein ingredients, peptide sources, instruments, etc., can solve the problems of reducing the resulting kinase activity and antisense nucleic acids interfering with the expression of mrna into protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
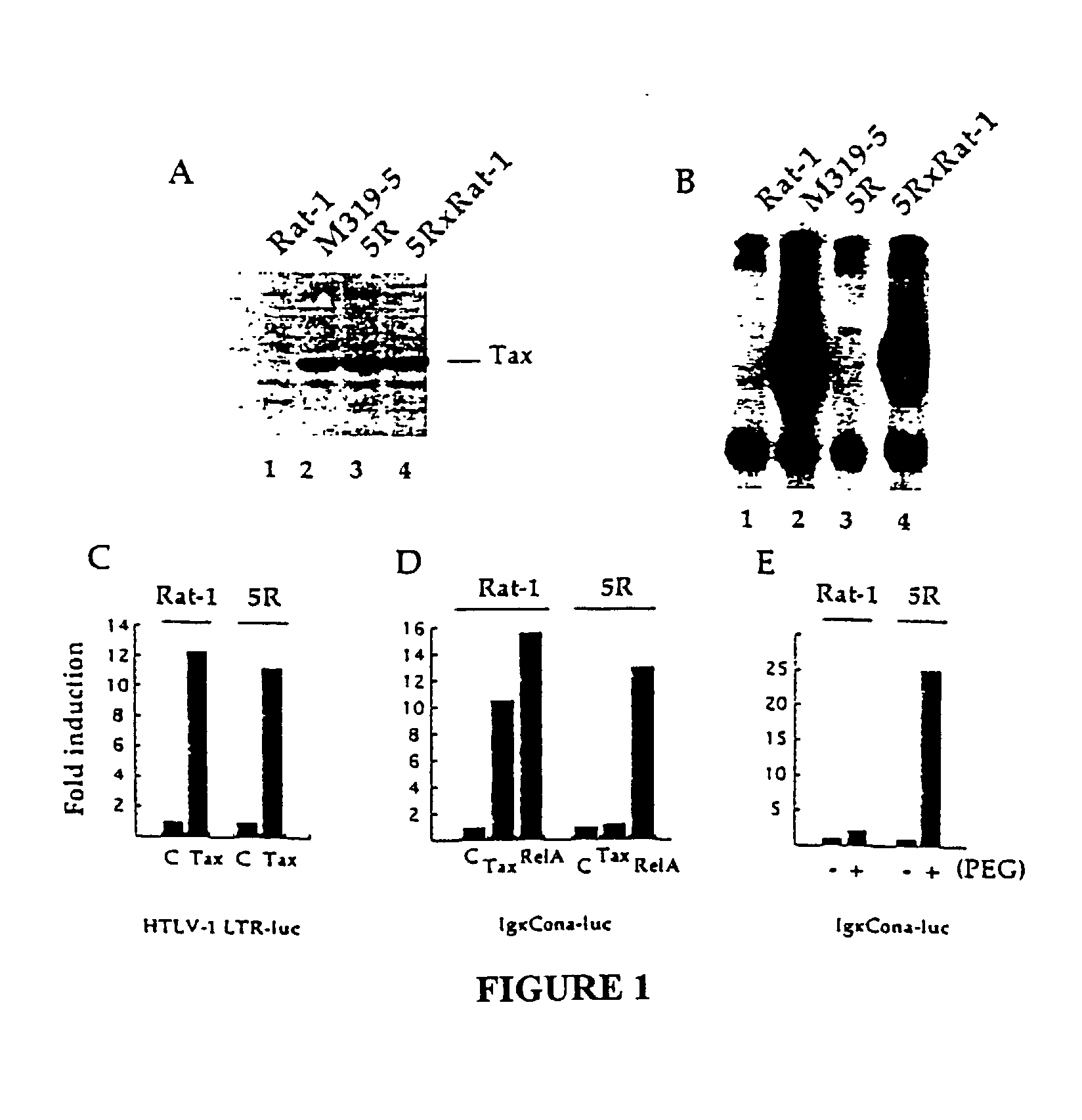
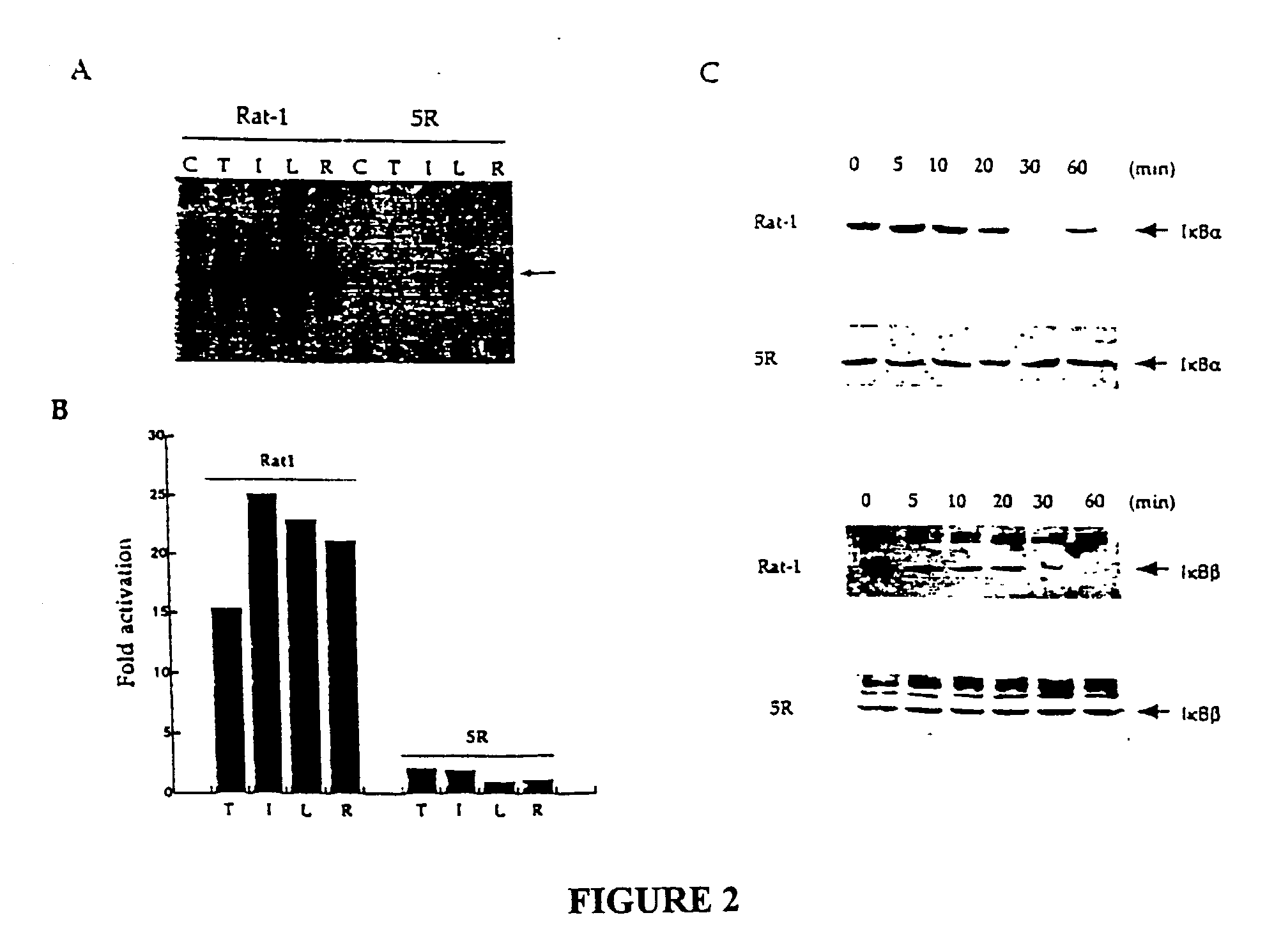
[0195] Materials and Methods
[0196] Cells and transfections. The 70Z / 3 murine pre-B cell line and the NF-.kappa.B unresponsive mutant 1.3E2 were maintained in RPMI medium supplemented with 10% foetal calf serum and 50 .mu.M .beta.-mercaptoethanol. 70Z / 3 and 1.3E2 cells were transiently transfected as described (Courtois et al., 1997). Isolated stable clones were prepared as described (Whiteside et al., 1995). Rat-1 and 5R cells were grown in DMEM supplemented with 10% foetal calf serum and transfected using the calcium phosphate coprecipitation method. For measurement of luciferase activity in transiently transfected Rat-1 or 5R cells, approximately 2.times.10.sup.5 cells were transfected with 0.25 .mu.g of a reporter plasmid, 0.25 .mu.g of EF1-lacZ plasmid and 1 .mu.g of either vector or effector plasmid. Cells were harvested 40 to 45 hours after transfection. The amount of lysate used for luciferase assay was determined on the basis of .beta.-galactosidase activity. The results sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| Tm | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com