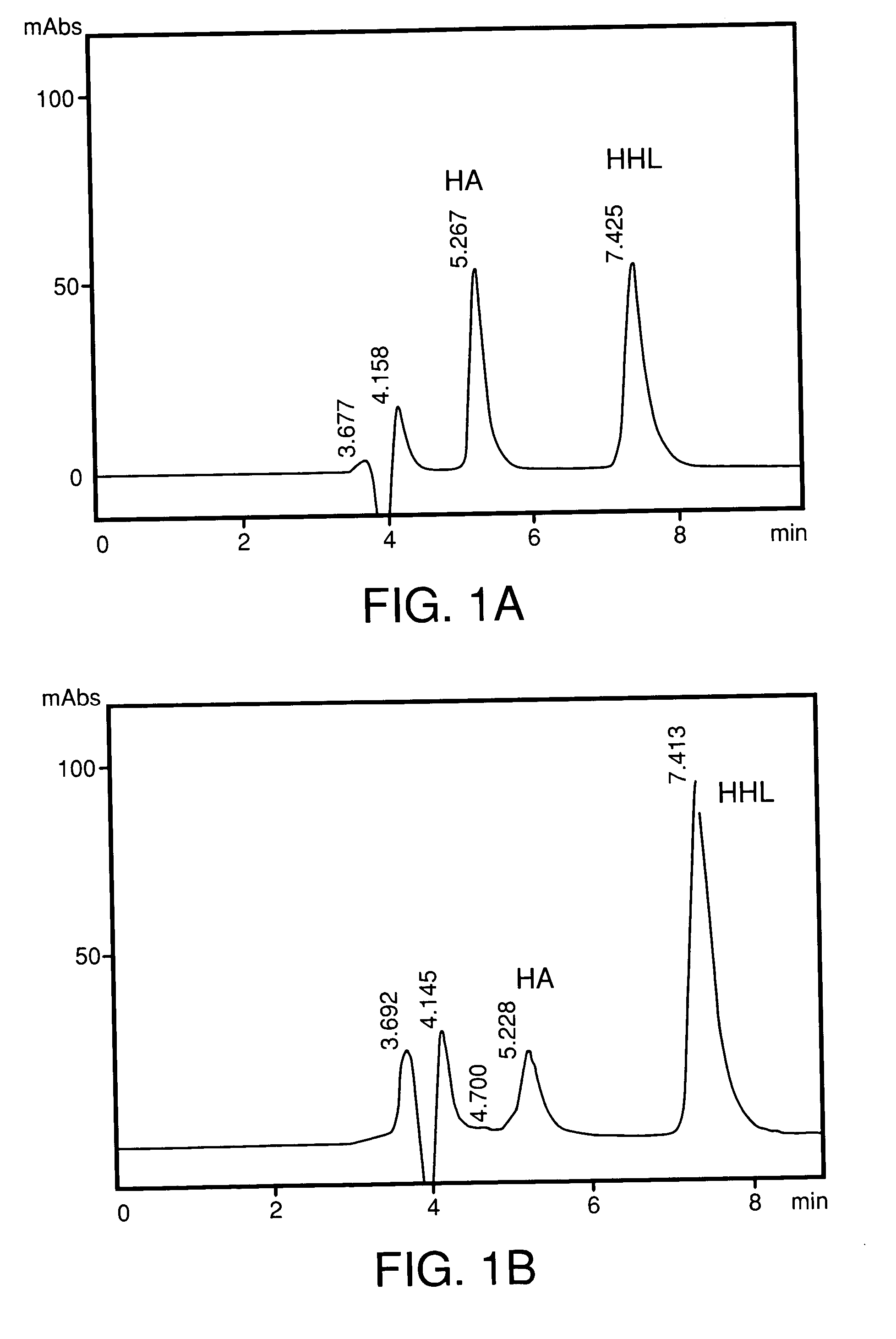
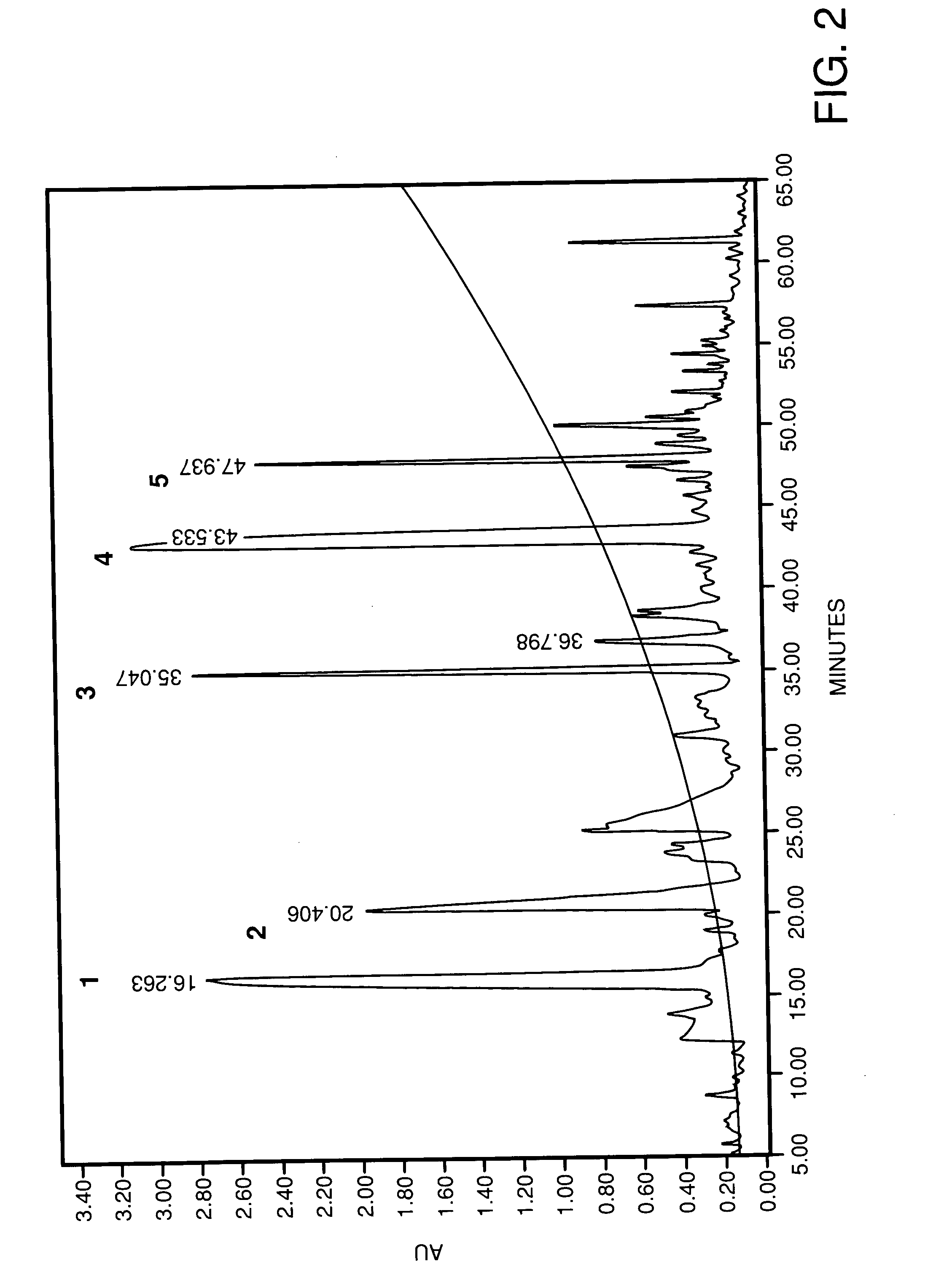
Process for the preparation of angiotensis converting enzyme (ACE) inhibitors and its use
a technology angiotensis, which is applied in the field of process for the preparation of angiotensis converting enzyme (ace) inhibitors, can solve the problems of limiting factors, extensive and expensive protocols, and high potency of synthetic ace inhibitors, and achieves potent ace inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
[0058] 500 mg of isolated glycinin was dissolved in 2 mL of Tris-HCl buffer, pH 8.2 and incubated at 37.degree. C. for 10 mins. 10 mg of bovine trypsin (1600 units / mg protein) was added and incubated for 4 h at 37.degree. C. This was followed by the addition of a second aliquot of 10 mg bovine trypsin and further incubated 12 h at 37.degree. C. The reaction was stopped by adding 100% TCA (w / w) to a final concentration of 5%. Centrifuged supernatant is used as peptide source for ACE inhibitory peptides. The IC.sub.50 value for inhibition of porcine kidney ACE was 18.37 .mu.g N.sub.2 equivalence.
example 3
[0059] 500 mg of isolated glycinin was dissolved in 2 mL of Tris-HCl buffer, pH 8.2 and incubated at 37.degree. C. for 10 mins. 10 mg of bovine chymotrypsin (2,168 units / mg protein) was added and incubated for 4 h at 37.degree. C. This was followed by the addition of a second aliquot of 10 mg bovine chymotrypsin and further incubated 12 h at 37.degree. C. The reaction was stopped by adding 100% TCA (w / w) to a final concentration of 5%. Centrifuged supernatant is used as peptide source for ACE inhibitory peptides. The IC.sub.50 value for inhibition of porcine lung ACE was 29.0 .mu.g N.sub.2 equivalence.
example 4
[0060] 500 mg of isolated glycinin was dissolved in 2 mL of sodium phosphate buffer, pH 6.0 and incubated at 50.degree. C. for 10 mins. 220 units of ginger protease was added and incubated for 4 h at 50.degree. C. This was followed by the addition of a second aliquot of 100 units of ginger protease and further incubated 12 h at 50.degree. C. The reaction was stopped by adding TCA to a final concentration of 5%. Centrifuged supernatant is used as peptide source for ACE inhibitory peptides. The IC.sub.50 value for inhibition of porcine kidney ACE was 33.57 .mu.g N.sub.2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| systolic blood pressure | aaaaa | aaaaa |
| blood pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com