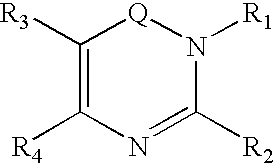
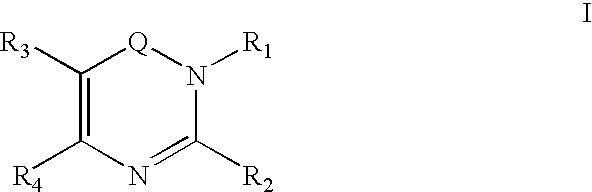
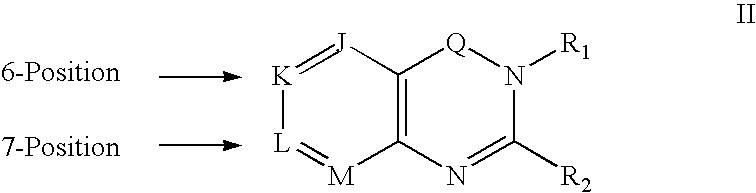
Dipeptidyl peptidase inhibitors
a technology of dipeptidyl peptides and inhibitors, which is applied in the field of dipeptidyl peptidase inhibitors, can solve the problems of short half-life of glp-1 (7-36), infertility and amenorrhea, and rapid degradation of glp-1 in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1b
2,4-Dichloro-quinazoline
[0807] 49
[0808] To 3.2 g of 1H-quinazoline-2,4-dione (1A) in 20 mL POCl.sub.3 was added 0.8 mL NN-dimethylaniline. The mixture was then heated at reflux for 16 hours. Excess POCl.sub.3 was removed in vacuo, providing crude product 1B.
example 1c
2-Chloro-3H-quinazolin-4-one
[0809] 50
[0810] A mixture of 20 mL of 1N NaOH, 20 mL of THF, and 2 g of 1B was stirred at room temperature under N.sub.2 for 4 hours. The solution was chilled and adjusted to pH 5 with AcOH. The solids that precipitated were filtered to give 1.62 g (90%) of product 1C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com