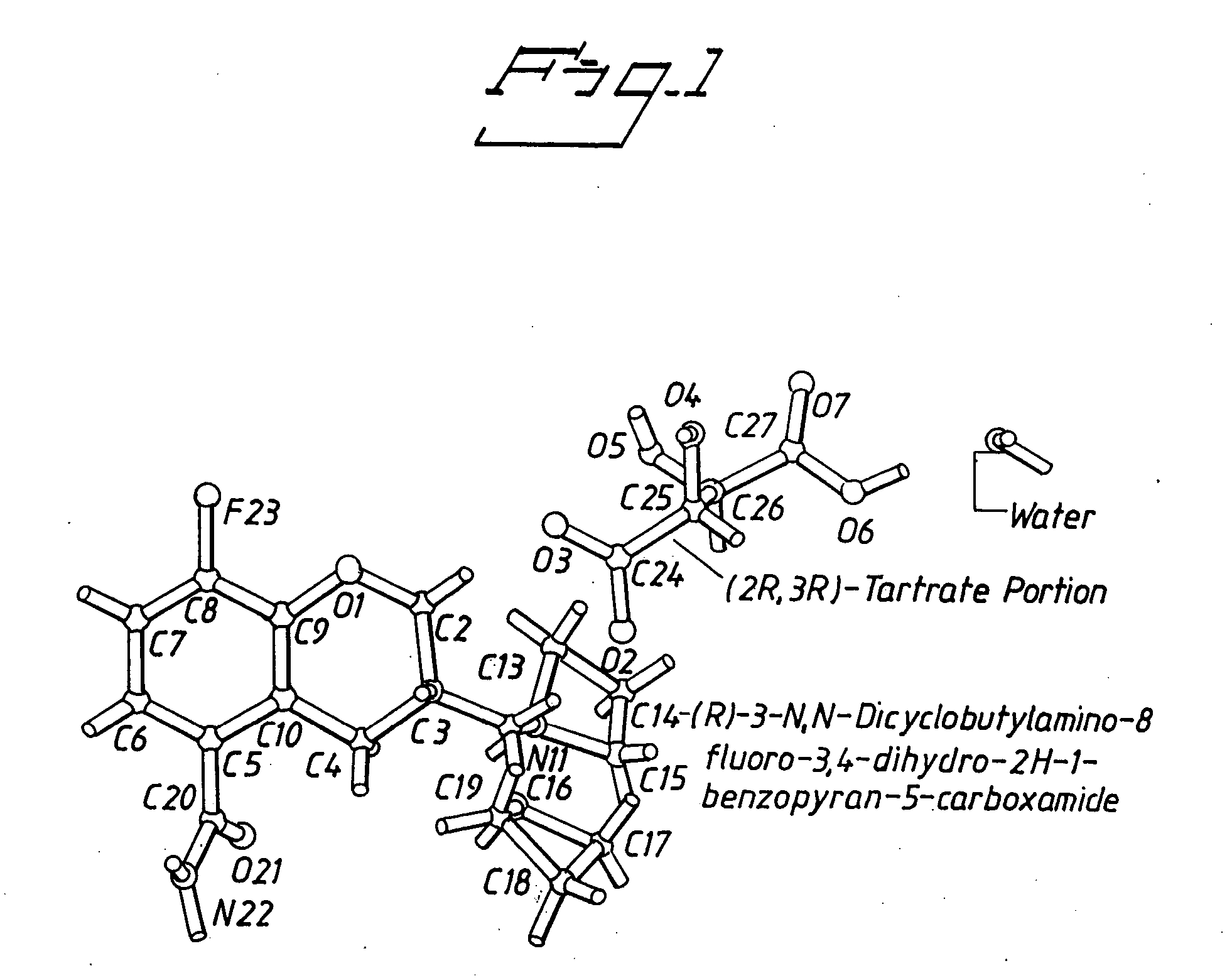
Tartrate salts of (R)-3-N,N-dicyclo-butylamino-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide
a technology of tartrate salt and dihydro-2h-1, which is applied in the field of salts, can solve the problems of the property of absorbing water and other problems, and achieve the effect of good solubility and dissolution properties of tartrate salt and easy work
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
(R)-3-N,N-dicyclobutylamino-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide Hydrogen (2R,3R)-Tartrate Monohydrate
[0034] (R)-3-N,N-Dicyclobutylamino-8-fluoro-3,4-dihydro-2H-1-benzopyran-5-carboxamide (2.0 g, 6.3 mmol) was dissolved in tetrahydrofuran (5 mL) by heating and the solution was diluted with diethyl ether (400 mL). To this solution was added a solution of (2R, 3R)-tartaric acid made by dissolving 1.1 g (6.9 mmol) of (2R, 3R)-tartaric acid in tetrahydrofuran (15 mL) and diluting with diethyl ether (300 mL). The clear solution obtained was allowed to stand in the refrigerator over the weekend. The crystalline solid obtained was filtered and recrystallized from 1.5% aqueous acetone (400 mL) to give of the title compound 2.6 g sparkly crystals (85% yield). Mp. 174-180° C. (DSC). Anal. Calcd. for C22H25FN2O9: C, 54.3; H, 6.4; N, 5.8. Found: C, 54.4; H, 6.3; N, 5.6.
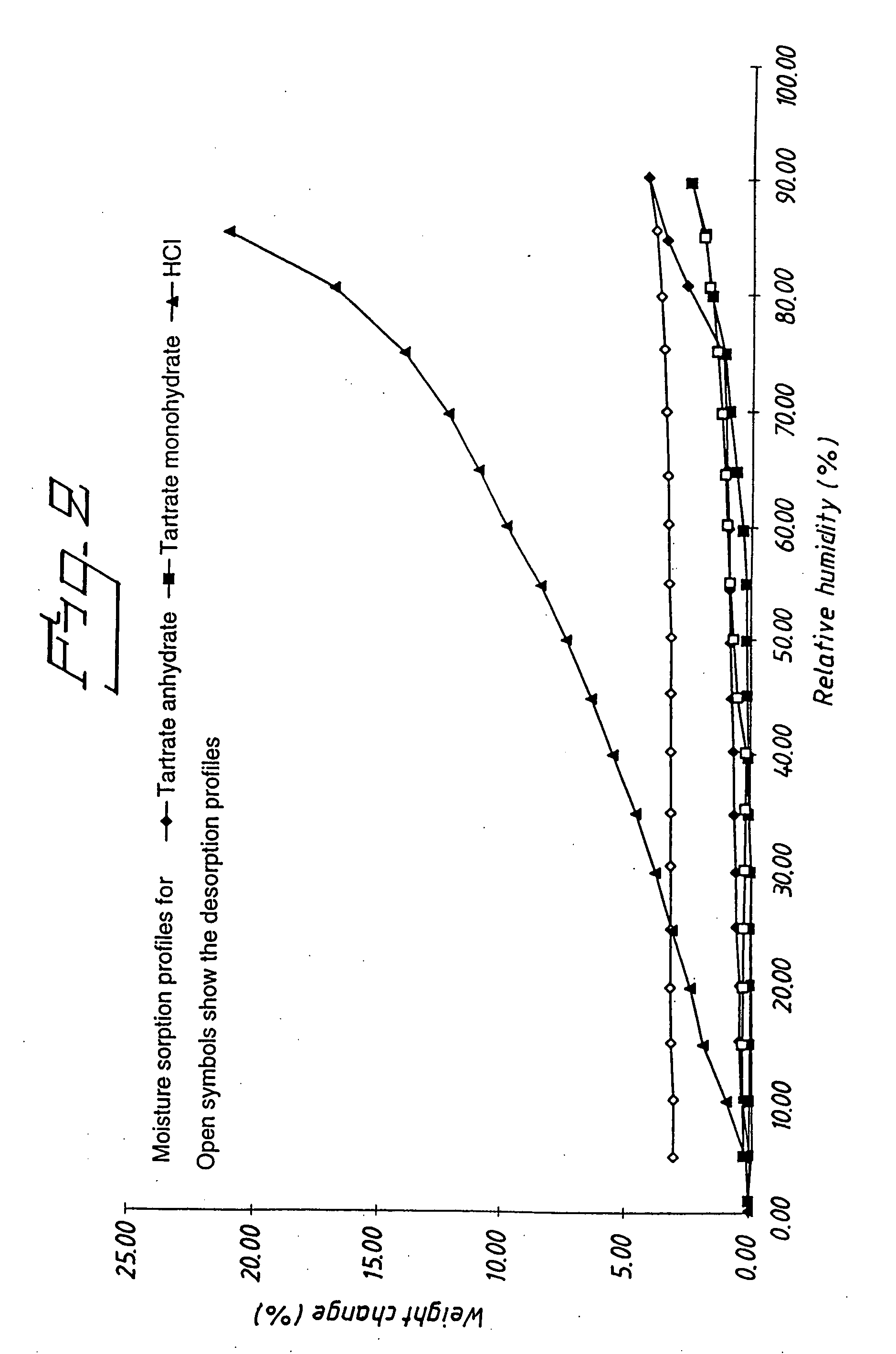
Analytical Test Method Used on the Products Obtained in Examples 1 and 2
[0035] The melting point (Mp) was meas...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperatures | aaaaa | aaaaa |
| RH | aaaaa | aaaaa |
| cognitive disorder | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com