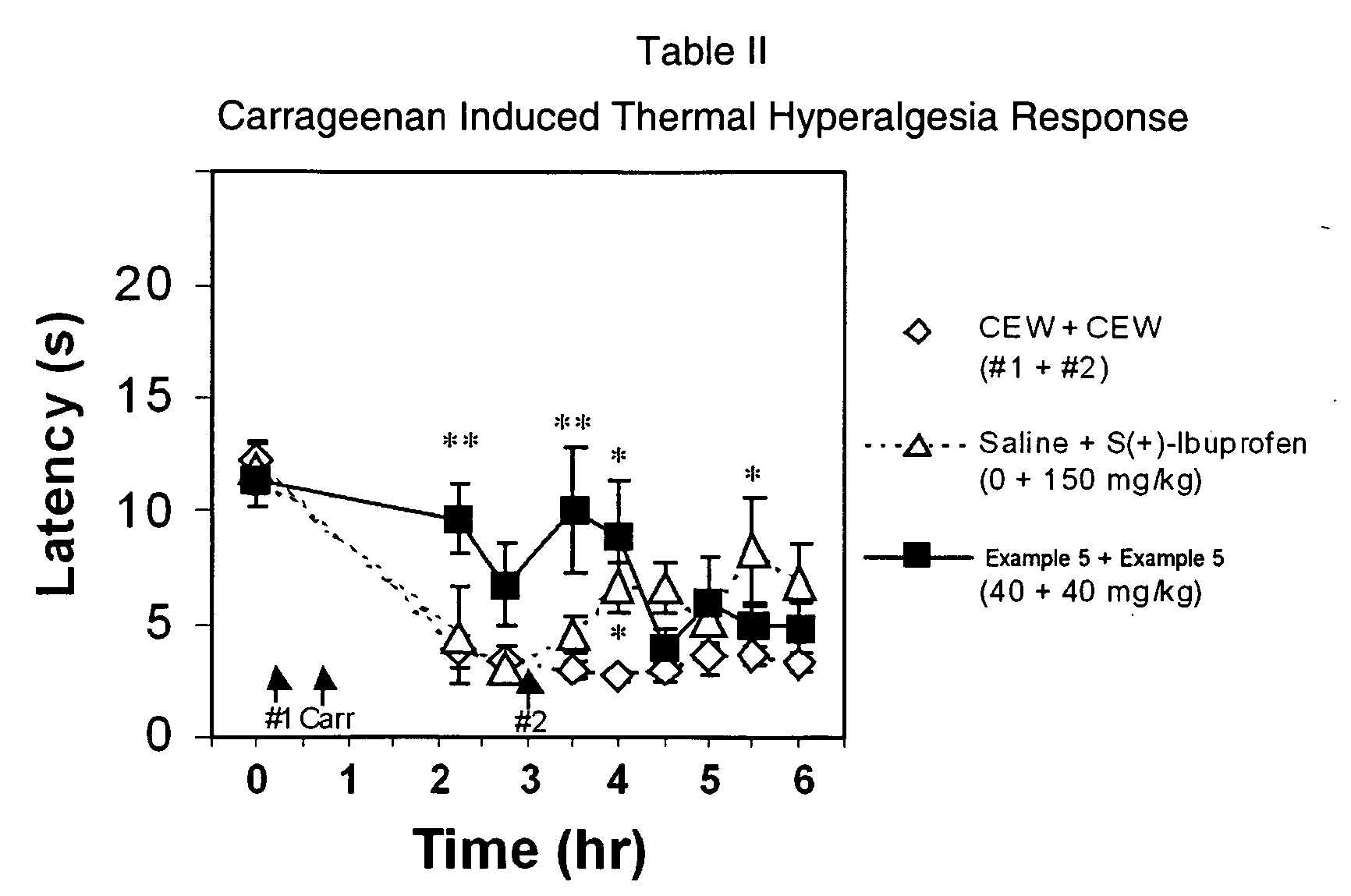
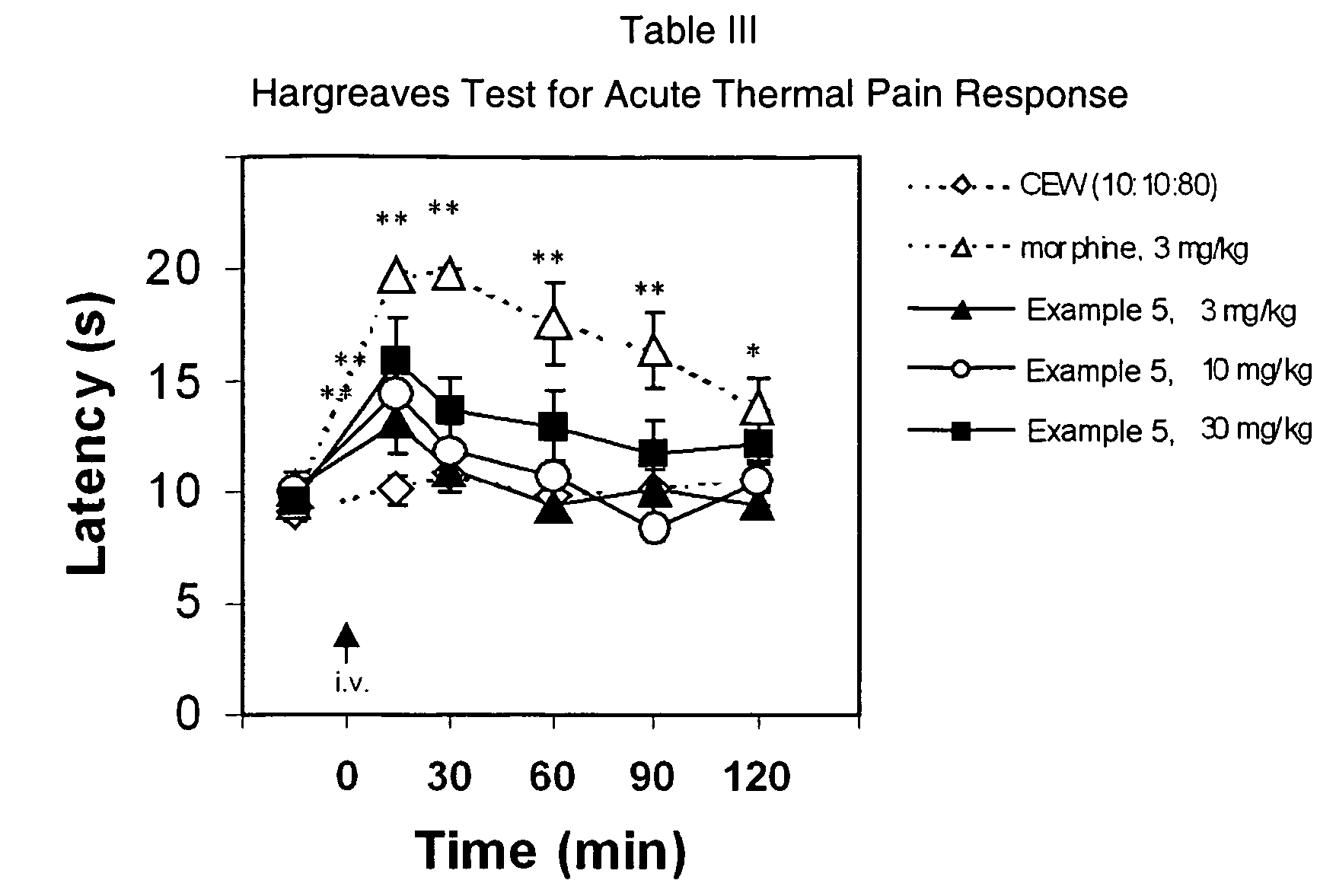
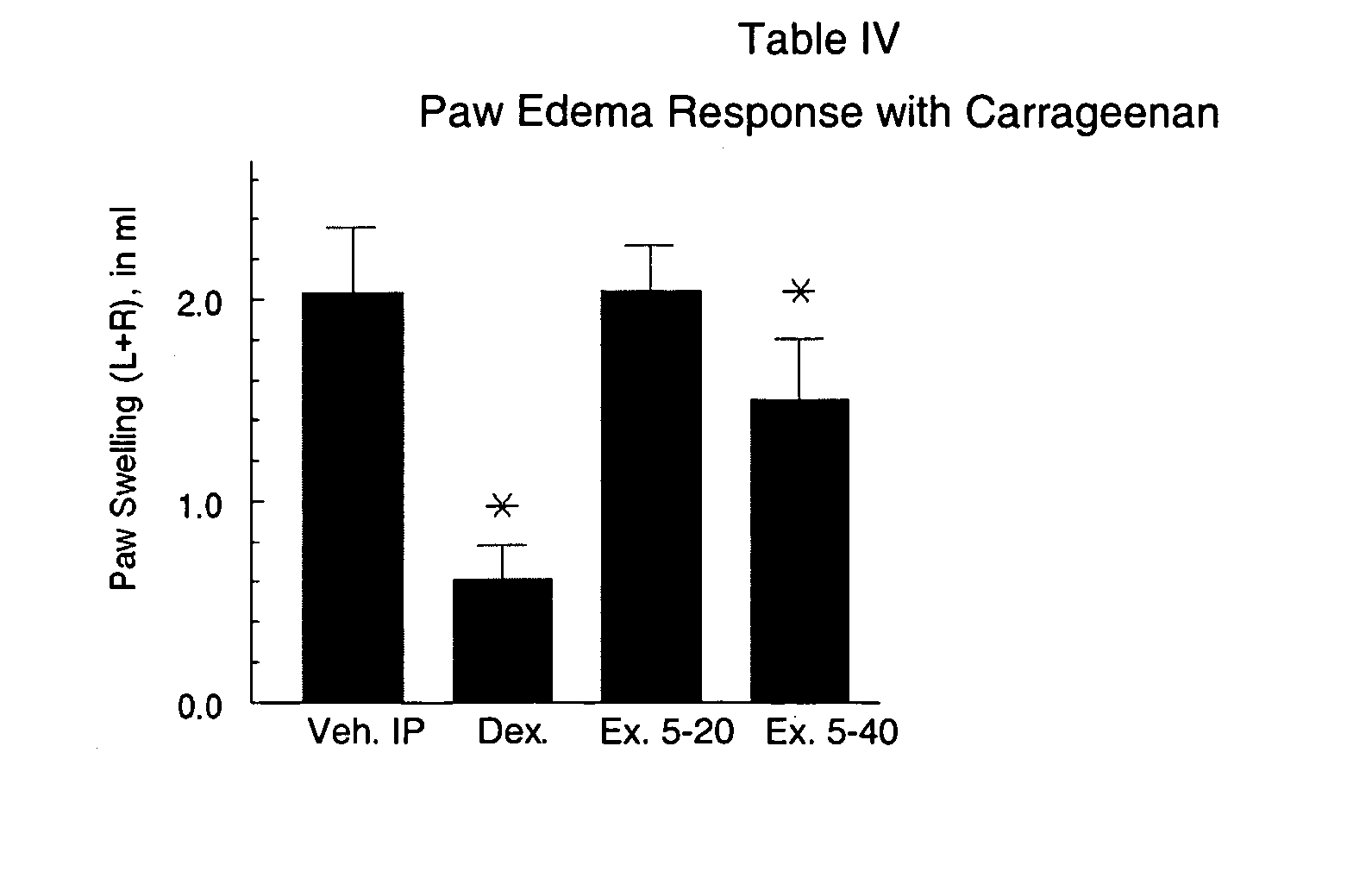
(Oxime)carbamoyl fatty acid amide hydrolase inhibitors
a technology of carbamoyl fatty acid amide and inhibitor, which is applied in the direction of biocide, drug composition, metabolic disorder, etc., can solve the problems of slow synthesis of drugs aimed at increasing the synthesis of these compounds, limited effective pain treatment with current therapies, and low synthesis rate of drugs. achieve the effect of modifying feeding behavior
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0268]
[0269] 3-Pyridinecarboxaldehyde, O-[[[4-(Heptyloxy)phenyl]amino]-carbonyl]oxime (Scheme 1, Compound D) To a solution of 4-heptyloxybenzoic acid (0.20 g, 0.85 mmol) and Et3N (0.18 g, 1.8 mmol) in toluene (5 mL) was added diphenylphosphoryl azide (0.322 g, 1.2 mmol). The resultant mixture was stirred at r.t. for 10 min. and then at 105° C. under N2 for 60 min. After the mixture was cooled to r.t., 3-pyridinealdoxime (0.20 g, 1.7 mmol) was added. The reaction mixture was stirred at r.t. for 10 min. and then at 80° C. for 1 h. The product (free base) was purified by flash chromatography (SiO2: EtOAc / Hexanes) and then was dissolved in a solution of TFA in THF (5.0 mg / mL, 17.8 mL, 0.78 mmol). Removal of solvent provided this compound (TFA salt) as a pale yellow solid (0.244 g, 0.52 mmol, 61% yield). 1H NMR (DMSO-d6) δ 9.72 (s, 1H), 8.99 (s, 1H), 8.72 (m, 2H), 8.32 (d, 1H, J=9.0 Hz) 7.56 (m, 1H), 7.40 (d, 2H, J=8.5 Hz), 6.91 (d, 2H, J=6.5 Hz), 3.92 (t, 2H, J=6.5 Hz), 1.72 (m, 2H), 1....
example 2
[0270]
[0271] (4-Butoxyphenyl)methylcarbamic acid, 3-pyridinyl ester (Scheme 1, Compound D) Prepared as described for the example above. 1H NMR (DMSO-d6) δ 8.51 (m, 3H), 7.75 (m, 1H), 7.55 (m, 1H), 7.24 (d, 2H, J=9.0 Hz), 6.91 (d, 2H, J=8.7 Hz), 4.22 (d, 2H, J=6.0), 3.92 (t, 2H, J=6.6 Hz), 1.70 (m, 2H), 1.36 (m, 2H), 0.92 (t, 3H, J=7.5 Hz); Anal. Calcd for C17H20N2O3.1.55 C2F3O2H1: C, 50.64; H, 4.56; N, 5.88. Found: C, 50.49; H, 4.58, N, 5.73. Mass Spec: 301.17 (MH+).
example 3
[0272]
[0273] 3-Pyridinecarboxaldehyde, O-[[[4-(undecyloxy)phenyl]amino]-carbonyl]oxime (Scheme 1, Compound D) Prepared as described for the example above. 1H NMR (DMSO-d6) δ 9.72 (s, 1H), 8.99 (s, 1H), 8.72 (m, 2H), 8.32 (d, 1H, J=9.0 Hz), 7.56 (m, 1H), 7.40 (d, 2H, J=8.5 Hz), 6.91 (d, 2H, J=6.5 Hz), 3.92 (t, 2H, J=6.5 Hz), 1.72 (m, 2H), 1.32 (m, 16 H), 0.87 (t, 3H, J=7.0 Hz). Mass Spec: 412.33 (MH+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com