Humanised antibodies
a technology of humanized antibodies and antibodies, applied in the field of humanised antibody molecules, can solve the problems of inherently limited use of rodent mabs as therapeutic agents in humans, and affecting the effect of immune respons
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
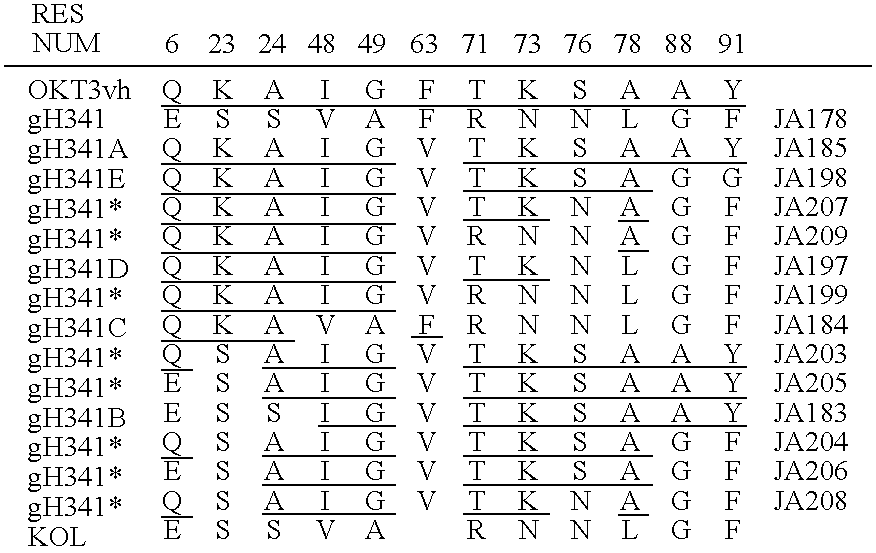
CDR-Grafting of OKT3
Material and Methods
1. Incoming Cells
[0142] Hybridoma cells producing antibody OKT3 were provided by Ortho (seedlot 4882.1) and were grown up in antibiotic free Dulbecco's Modified Eagles Medium (DMEM) supplemented with glutamine and St foetal calf serum, and divided to provide both an overgrown supernatant for evaluation and cells for extraction of RNA. The overgrown supernatant was shown to contain 250 ug / mL marine IgG2a / kappa antibody. The supernatant was negative for marine lambda light chain and IgG1, IgG2b, IgG3, IgA and IgM heavy chain. 20 mL of supernatant was assayed to confirm that the antibody present was OKT3.
2. Molecular Biology Procedures
[0143] Basic molecular biology procedures were as described in Maniatis et al (ref. 9) with, in some cases, minor modifications. DNA sequencing was performed as described in Sanger et al (ref. 11) and the Amersham International Plc sequencing handbook. Site directed mutagenesis was as described in Kramer et...
example 2
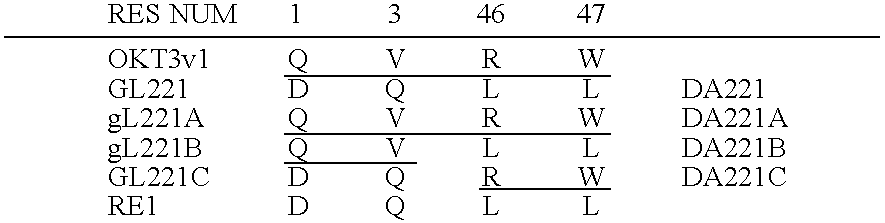
CDR-Grafting of a Murine Anti-CD4 T Cell Receptor Antibody, OKT4A
[0241] Anti OKT4A CDR-grafted heavy and light chain genes were prepared, expressed and tested substantially as described above in Example 1 for CDR-grafted OKT3. The CDR grafting of OKT4A is described in detail in Ortho patent application PCT / GB 90 . . . of even date herewith entitled “Humanised Antibodies”. The disclosure of this Ortho patent application PCT / GB 90 . . . is incorporated herein by reference. A number of CDR-grafted OKT4 antibodies have been prepared. Presently the CDR-grafted OKT4A of choice is the combination of the grafted light chain LCDR2 and the grafted heavy chain HCDR10.
The Light Chain
[0242] The human acceptor framework used for the grafted light chains was RE1. The preferred LCDR2 light chain has human to mouse changes at positions 33, 34, 38, 49 and 89 in addition to the structural loop CDRs. Of these changed positions, positions 33, 34 and 89 fall within the preferred extended CDRs of the...
example 3
CDR-Grafting of an Anti-Mucin Specific Murine Antibody, B72.3
[0249] The cloning of the genes coding for the anti-mucin specific murine monoclonal antibody B72.3 and the preparation of 572.3 mouse-human chimeric antibodies has been described previously (ref. 13 and WO 89 / 01783). CDR-grafted versions of B72.3 were prepared as follows.
(a) B72.3 Light Chain
[0250] CDR-grafting of this light chain was accomplished by direct transfer of the murine CDRs into the framework of the human light chain RE1. The regions transferred were:
CDR NumberResidues124-34250-56390-96
[0251] The activity of the resulting grafted light chain was assessed by co-expression in COS cells, of genes for the combinations: [0252] B72.3 cH / B72.3 cL [0253] and B72.3 cH / B72.3 gL
[0254] Supernatants were assayed for antibody concentration and for the ability to bind to microtitre plates coated with mucin. The results obtained indicated that, in combination with the B72.3 cH chain, B72.3 cL and B72.3 gL had similar bi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity constant | aaaaa | aaaaa |
| binding affinity | aaaaa | aaaaa |
| structure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com