Soluble GlcNAc phosphotransferase
a phosphotransferase and soluble technology, applied in the direction of transferases, peptide/protein ingredients, peptide sources, etc., can solve the problems of inability to degrade, inability to efficiently subject recombinant soluble glcnac-phosphotransferase to post-translational proteolytic cleavage, and inability to degrade, etc., to achieve high mannose structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
MATERIALS AND METHODS
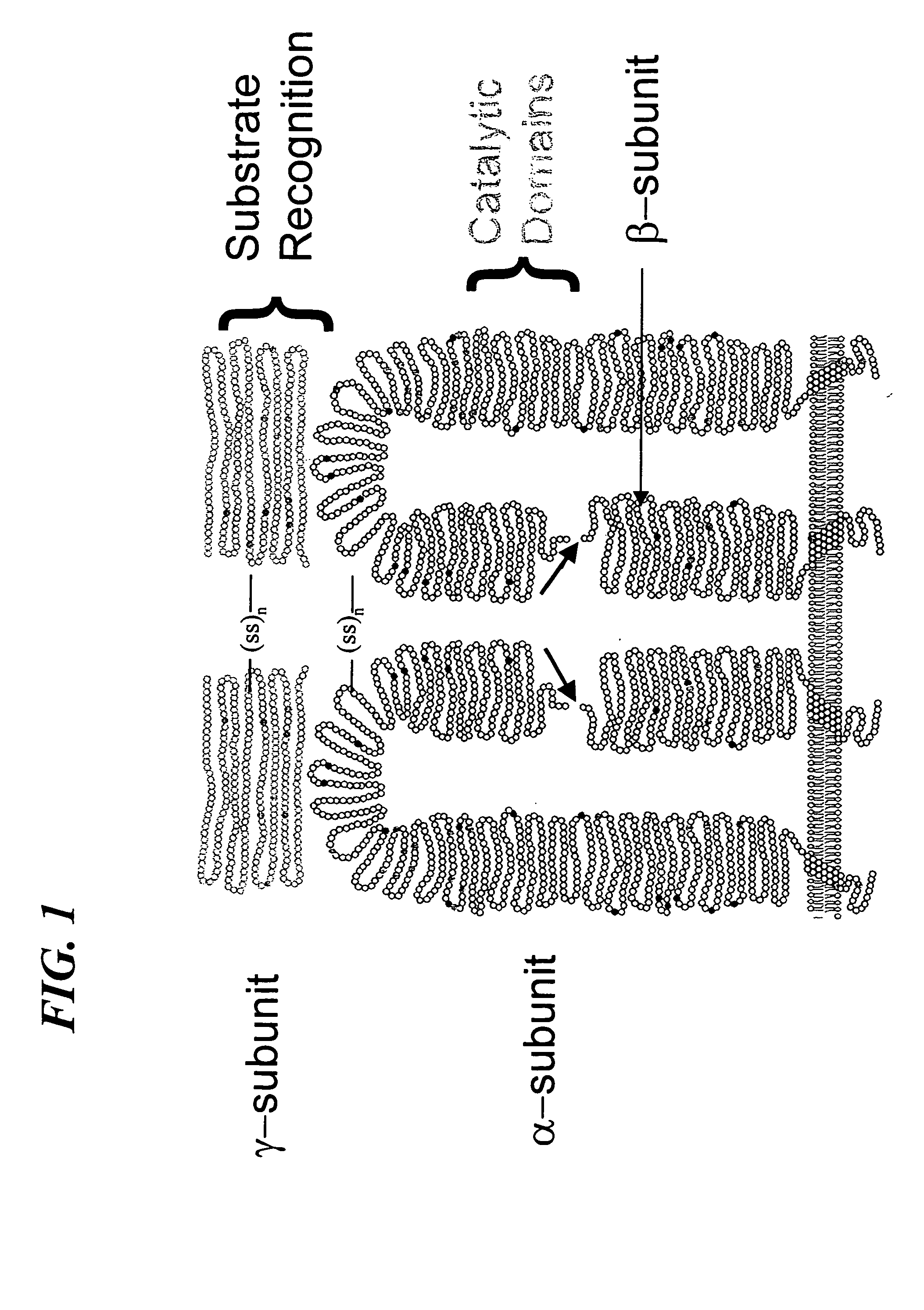
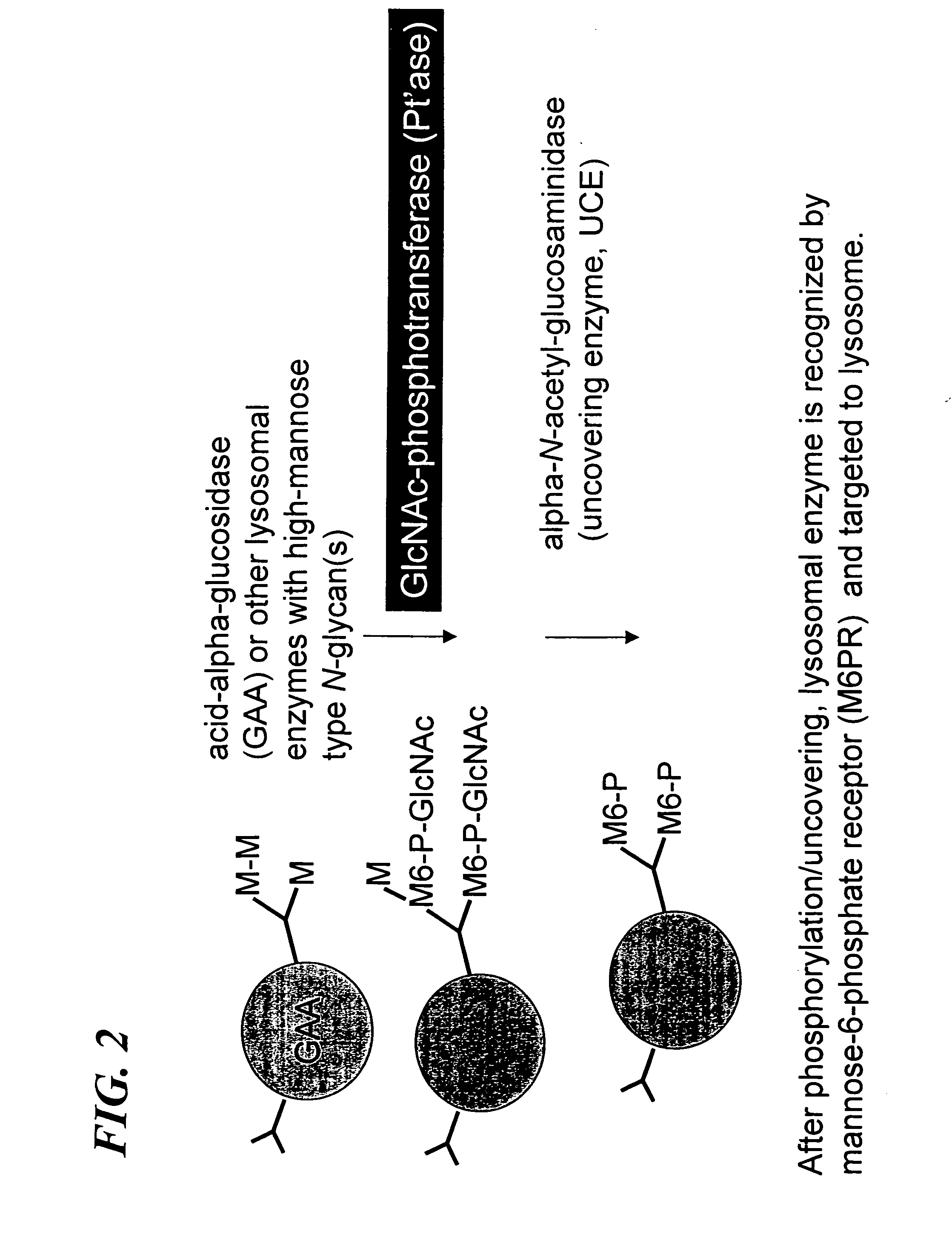
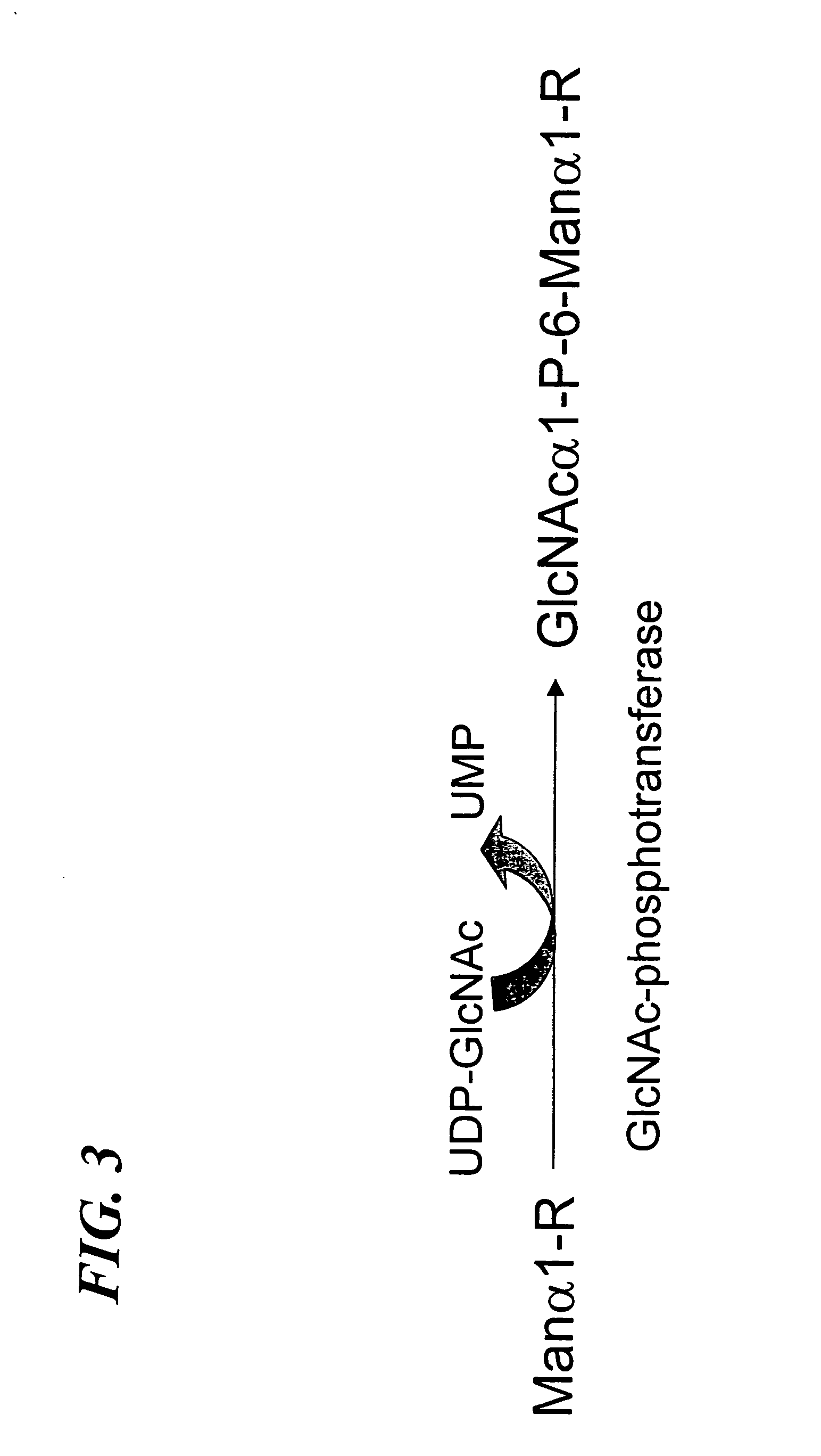
[0074] Construction of the Furin-cleavage site containing α / β subunit of GlcNAc-phosphotransferase—The molecular cloning and expression of wild type human UDP-N-acetylglucosamine: lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase (GlcNAc-phosphotransferase) is described in U.S. Ser. No. 09 / 636,060 and PCT / US00 / 21970, incorporated herein by reference. Also, the construction and expression of recombinant soluble human UDP-N-acetylglucosamine: lysosomal-enzyme-N-acetylglucosamine-1-phosphotransferase (GlcNAc-phosphotransferase) is described in U.S. Ser. No. 09 / 636,060 and PCT / US00 / 21970, incorporated herein by reference. The soluble GlcNAc-phosphotransferase α / βsubunit cDNA was contained within the Nhe I and Xba I site of pcDNA 6NV5 / His-A (Invitrogen). This plasmid, designated, pMK 52 was used as the starting material for the construction of the furin-cleavage site containing α / β subunit of recombinant soluble GlcNAc-phosphotransferase as shown in FIG. 7. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com