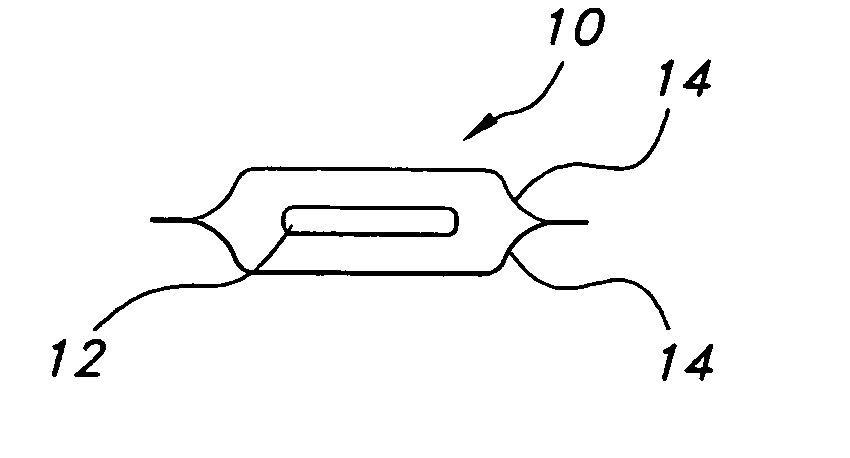
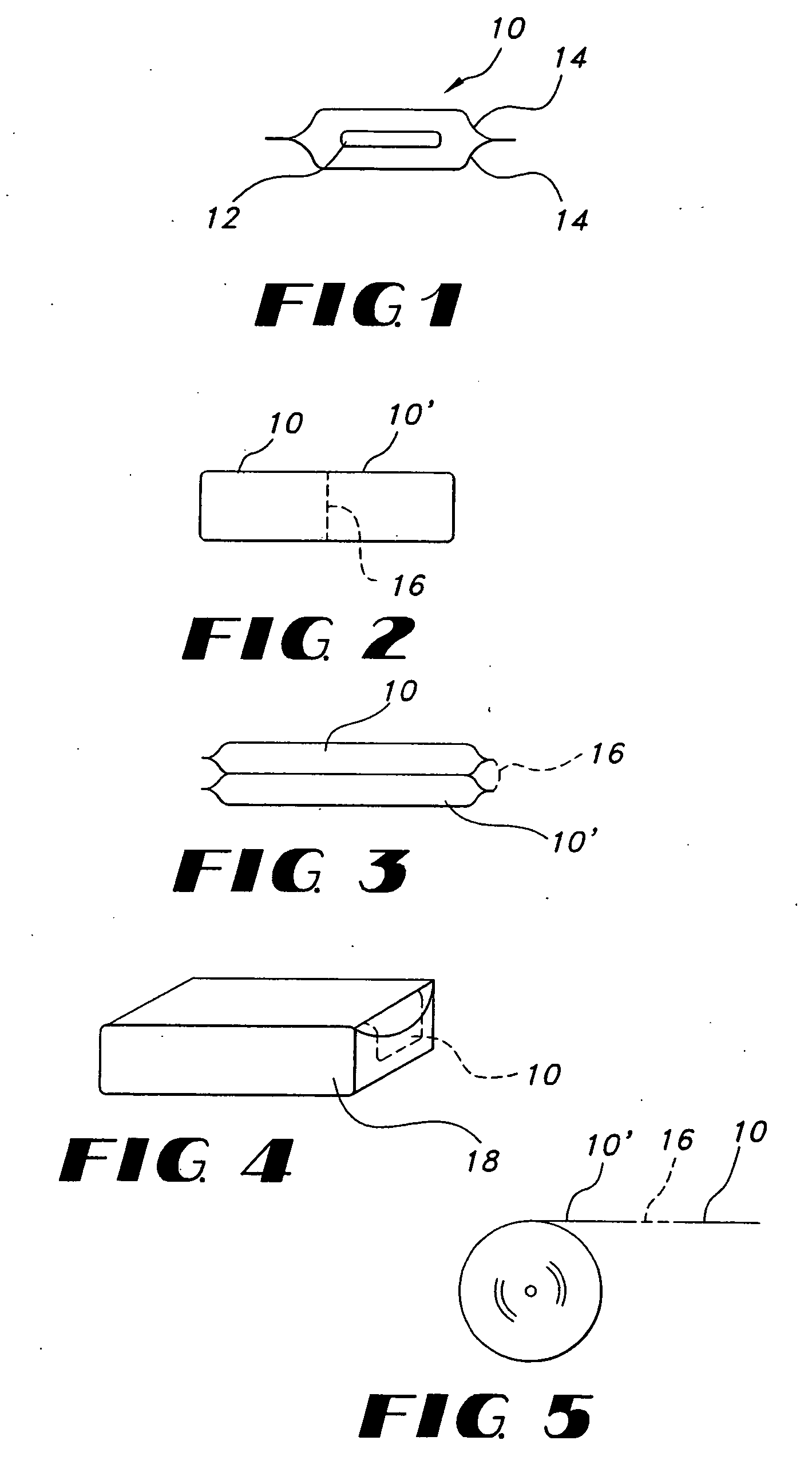
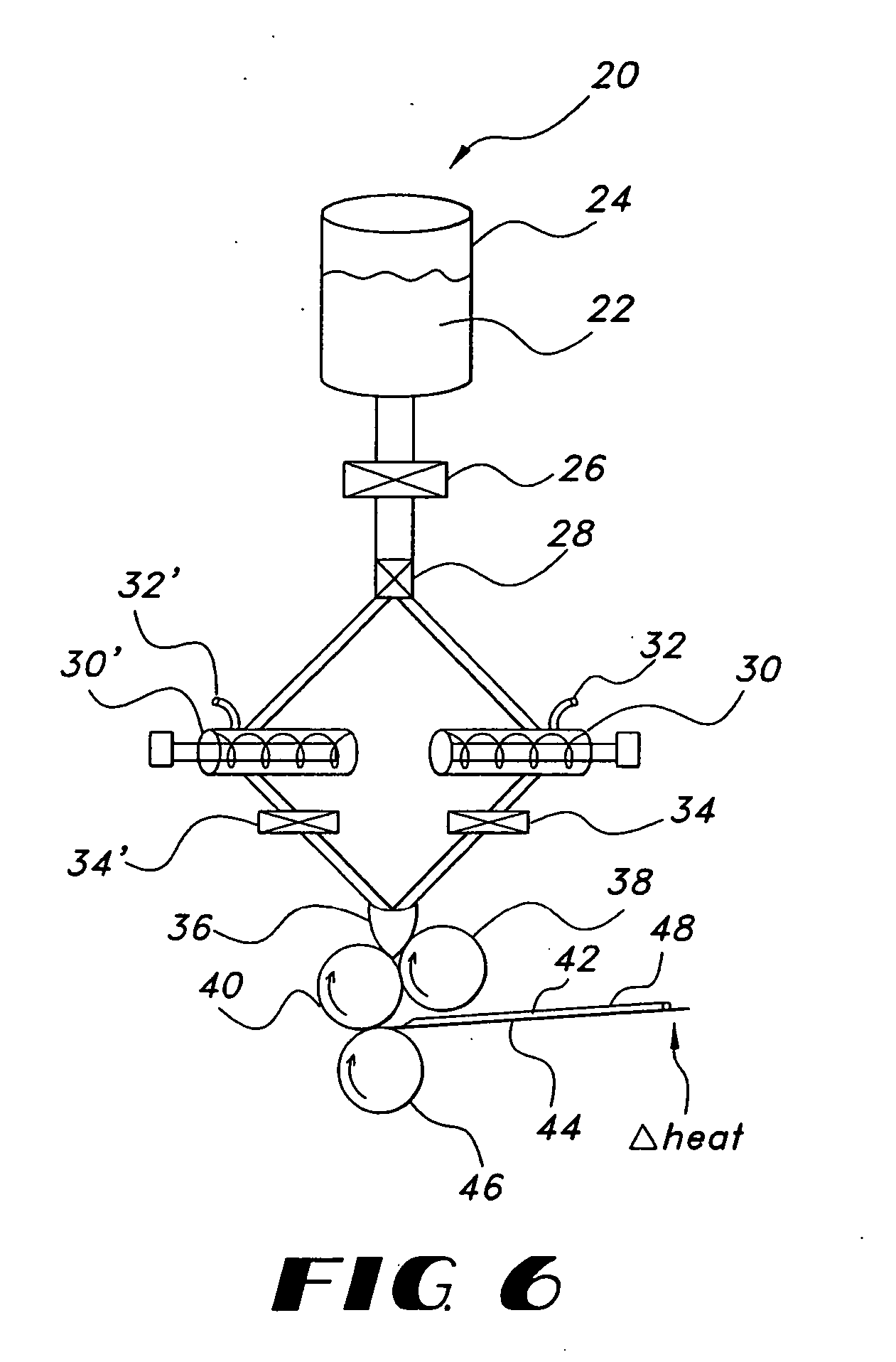
Thin film with non-self-aggregating uniform heterogeneity and drug delivery systems made therefrom
a thin film, non-self-aggregating technology, applied in the direction of respiratory disorder, digestive system, pharmaceutical non-active ingredients, etc., can solve the problem that the dosage form of pharmaceutical film has not been marketed largely to da
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0154] Water soluble thin film compositions of the present invention are prepared using the amounts described in Table 1.
TABLE 1Weight (g)IngredientABCDEFGHIHydroxypropylmethyl1.761.6332.003.6732.00cellulosePeppermint oil0.901.01.058.02.67Sweetener0.150.150.220.104.61.530.15Polyvinylpyrrolidone0.941.057.02.33Tween 8010.50.52.00.6511.801.350.511.80Simethicone20.20.20.150.301.800.210.21.80Listerine383.3583.35Methylcellulose6.0Cornstarch41.75Agar1.25Water42.2493.6339.22768.00280.088.24768.0Loratadine519.219.2Pullulan66.0Ibuprofen38.4
1Available from ICI Americas
2Available from OSI
3Available from Pfizer, Inc. including thymol (0.064%), eucalyptol (0.092%), methyl salicylate (0.060%), menthol (0.042%), water (up to 72.8%), alcohol (26.9%), benzoic acid, poloxamer 407, sodium benzoate, and caramel color
4Available from Grain Processing Corporation as Pure Cote B792
5Available from Schering Corporation as Claritin
6Available from Hayashibara Biochemical Laboratories, Inc., Japan
[0155] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight percent | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com