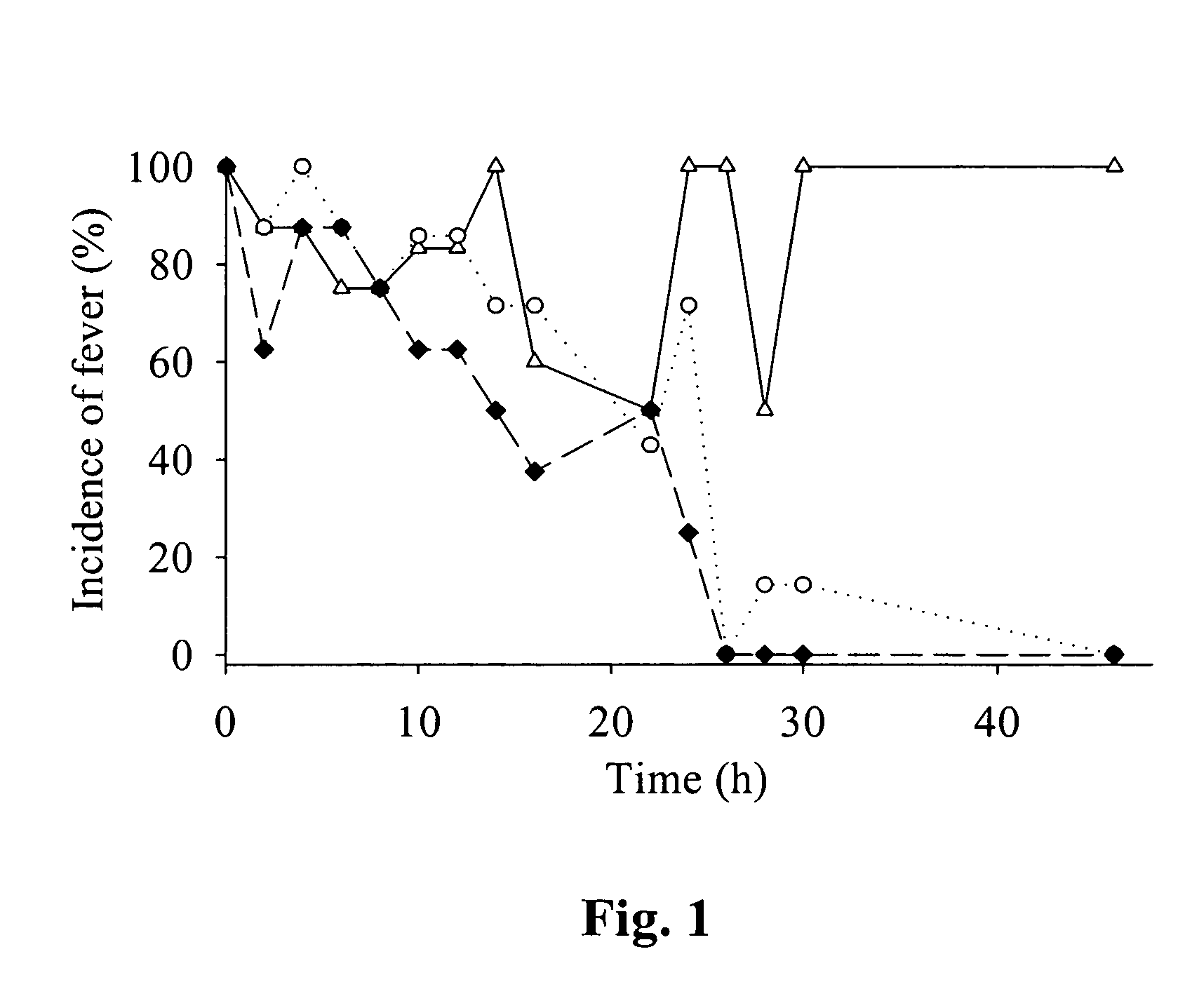
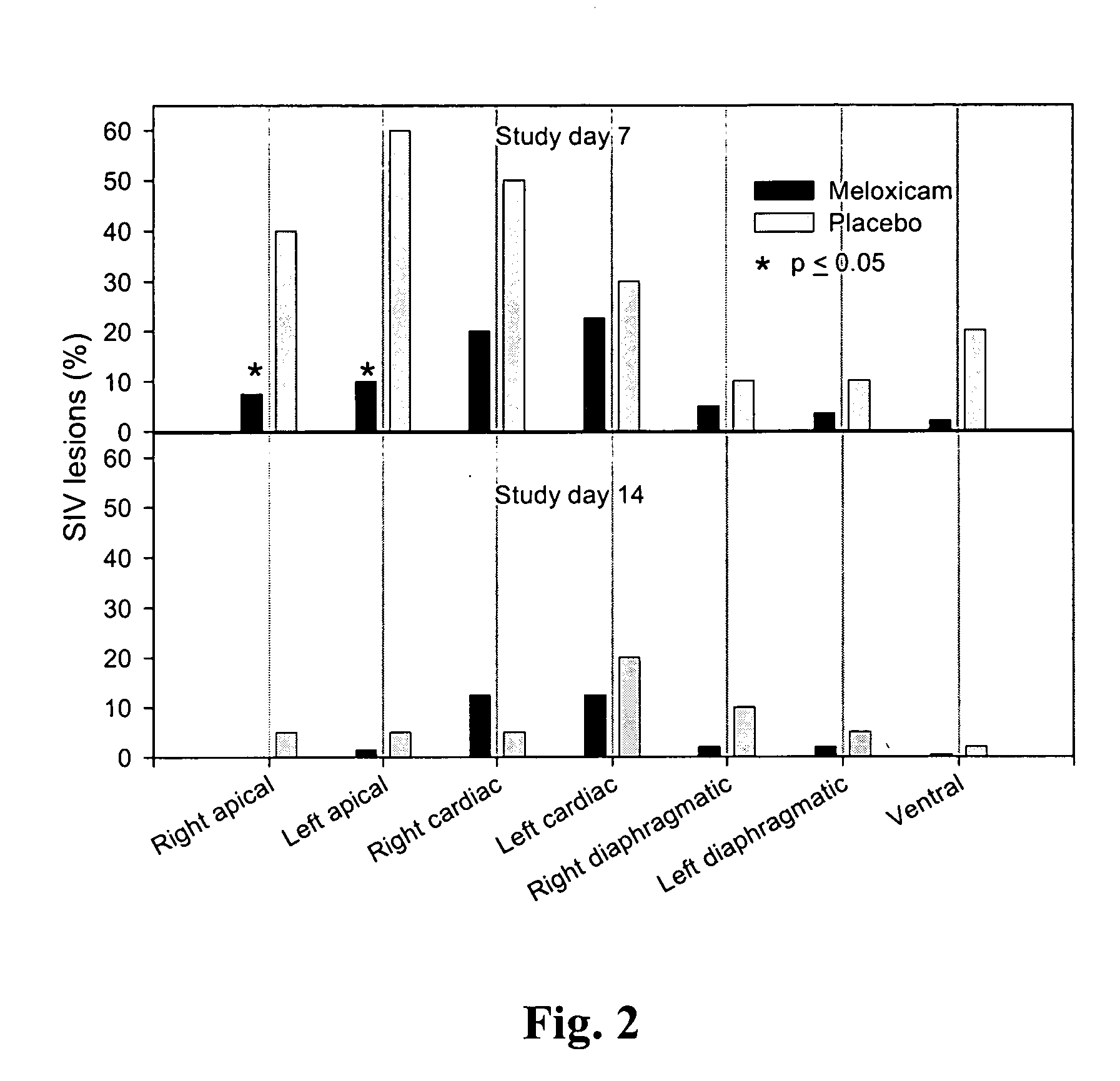
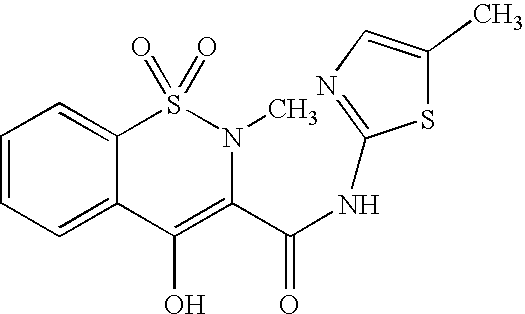
Meloxicam for the treatment of respiratory diseases in pigs
a technology of meloxicam and pigs, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, tetracycline active ingredients, etc., can solve the problems of almost entirely missing data on non-steroidal anti-inflammatory drugs in pigs, and no information on the use of meloxicam in pigs with respiratory diseases is publicly availabl
Inactive Publication Date: 2005-08-25
BOEHRINGER LNGELHEIM VETMEDICA GMBH
View PDF18 Cites 55 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
The patent text describes the use of meloxicam to treat or prevent respiratory diseases in pigs. The invention relates to a pharmaceutical composition containing meloxicam for this purpose. The text also describes a method of treating or preventing respiratory diseases in pigs by administering meloxicam. Additionally, the invention includes a veterinary preparation containing meloxicam and at least one antibiotic, as well as a ready-to-use two-component system for the treatment of respiratory diseases in pigs. The technical effects of the invention include improved treatment and prevention options for respiratory diseases in pigs and a more effective combination of meloxicam and antibiotics.
Problems solved by technology
However, if pigs have developed respiratory disease, they have to be treated.
67-74, Hatfield, U.K. Proc. Royal Vet. Coll.) focuses on pharmacokinetics used in the treatment of respiratory disease in cattle and pigs; however, non-steroidal anti-inflammatory drugs data for pigs was almost entirely lacking and only lists data for cattle including meloxicam.
However, to date no information on the use of meloxicam in pigs with respiratory disease is publicly available.
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
example a
0.6% Meloxicam Granules
[0037]
g / 100 gMeloxicam0.6Meglumine0.42Hydroxypropylmethylcellulose3.00Povidone2.00Glucose monohydrate93.98
example b
1.2% Meloxicam Granules
[0038]
g / 100 gMeloxicam1.2Meglumine0.84Hydroxypropylmethylcellulose3.00Collidone 252.0Glucose Monohydrate92.96
example c
0.6% Meloxicam Granules
[0039]
g / 100 gMeloxicam0.6Meglumine0.42Pharmacoat 6064.0Macrogol 60001.0Acesulfame K0.3Lactose93.68
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| half-time | aaaaa | aaaaa |
| half-time | aaaaa | aaaaa |
| rectal temperature | aaaaa | aaaaa |
Login to View More
Abstract
A method of treating or preventing a respiratory disease in a pig, the method comprising administering to the pig in need thereof an effective amount of meloxicam or a pharmaceutically acceptable salt thereof.
Description
RELATED APPLICATIONS [0001] This application claims priority to European Application No. EP 04 004 054.5, filed Feb. 23, 2004, which is hereby incorporated by reference. BACKGROUND OF THE INVENTION [0002] 1. Technical Field [0003] The invention relates to the use of meloxicam or a pharmaceutically acceptable salt thereof for preparing a pharmaceutical composition for the treatment or prevention of respiratory diseases in pigs. [0004] 2. Background Information [0005] Respiratory disease in pigs belongs to the most important health problems in swine production. Porcine respiratory disease is primarily caused by infectious agents, but environmental factors have a strong influence. The relevant pathogens include mycoplasmas, bacteria, and viruses (e.g., G. Christensen, V. Sorensen, and J. Mousing, Diseases of the Respiratory System, In: Diseases of Swine, B. E. Straw, S. D'Allaire, W. L. Mengeling, & D. J. Taylor (eds), Iowa State University Press, Ames, Iowa (1999) pp. 913-940). [0006]...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(United States)
IPC IPC(8): A61K31/5415A61K45/06
CPCA61K31/5415A61K45/06A61K31/65A61K2300/00A61P11/00A61P31/00A61P31/10A61P31/12A61P31/14
Inventor LANG, INGOPAPATSAS, IOANNIS
Owner BOEHRINGER LNGELHEIM VETMEDICA GMBH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com