Dual phase - PNA conjugates for the delivery of PNA through the blood brain barrier
a technology of pna and conjugates, which is applied in the direction of animal/human proteins, sugar derivatives, non-active ingredients of pharmaceuticals, etc., can solve the problems of limited therapeutic value, the protection function of the blood-brain barrier becomes, and the nutrient transporters in the bbb can only deliver low molecular weight substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
PC12 Cells
Uptake of Conjugated PNAs into the Neuronal Cell Line PC12:
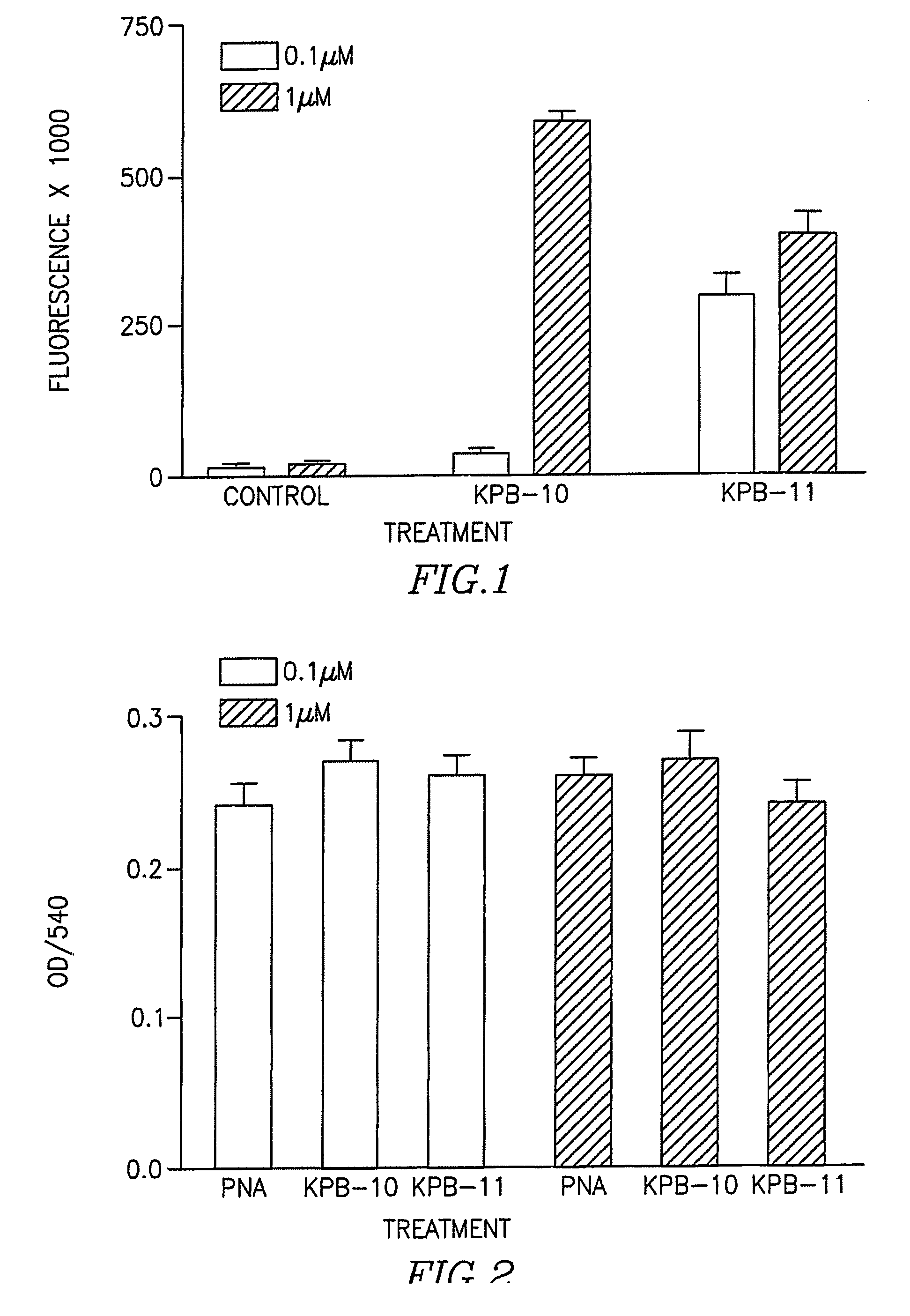
[0077] Cells were seeded on 96 well dishes coated with poly-1-lysine. Day after seeding medium was replaced with fresh medium without serum containing different concentrations (0.1-1 μM) of PNAs. Following 3 hours incubation medium was removed and cells were washed three times with acid wash solution and fluorescent determined (FIG. 1).
Control =TTT GCT CTT ACT CAT(SEQ ID No. 39)KBP10 =CHK6HC(SEQ ID No. 40)- TTT GCT CTT ACT CAT(SEQ ID No. 39)- THRPPMWSPVWP(SEQ ID No. 41)KBP11 =CHK6HC(SEQ ID No. 40)- TTT GCT CTT ACT CAT -(SEQ ID No. 39)HAIYPRH(SEQ ID No. 41)
As can be seen from FIG. 1, the uptake of either KBP10 and KBP11 into PC12 cells was much higher than the uptake of PNA alone.
Measurement of Cellular Toxicity:
[0078] PC12 cells were incubated with PNA or peptide-PNA conjugates for 48 hours. At the end of the incubation, cell morphology was examined by light microscopy. Medium was replaced with medium cont...
example 2
bEND3 Cell Line BBB Cellular Model
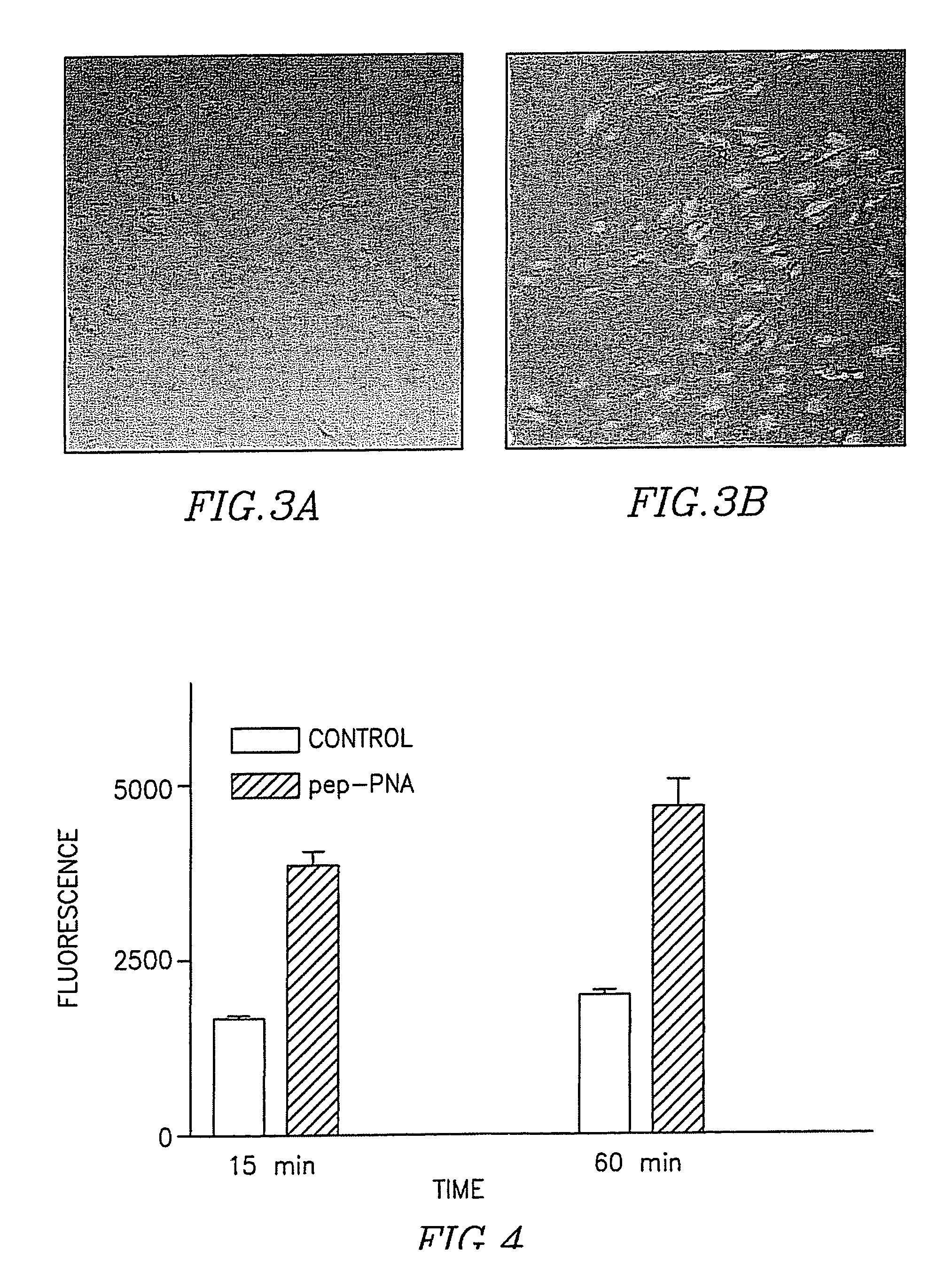
[0079] Uptake of fluorescence labeled PNA (TTT GCT CTT ACT CAT ) (SEQ ID. No. 39) or peptide-PNA to bEND3 (CHK6HC (SEQ ID. No. 40)-TTT GCT CTT ACT CAT-(SEQ ID. No. 39) HAIYPRH (SEQ ID. No. 41).
[0080] bEND3 cells were seeded on a poly-L-ornithine coated 35 mm dish. 24 hours following seeding the cell culture medium was replaced with DMEM containing 10 μM PNA or peptide-PNA. Cells were incubated for 4 hours. Following incubation cells were washed 3 times with PBS and medium was replaced with fresh DMEM cells as observed by confocal microscopy (FIG. 3). As can be clearly seen, the uptake of peptide-PNA is clearly observed whereas uptake of PNA alone is invisible.
example 3
NMB Cell Line
[0081] Uptake of PNA (GCAT) or conjugated peptide PNA (GCAT-THRPPMWSPVWP) (SEQ ID. No. 42) into the human neuronal cell line NMB. Cells were seeded on 96 well dishes coated with poly-1-ornithine. One day after seeding the medium was replaced with a fresh medium without serum, containing 1 micromolar PNAs. Following 15 or 60 min. incubation medium was removed and cells were washed three times with acid wash solution. Fluorescent was determined (FIG. 4).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric charge | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Nucleic acid sequence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com